Printable Vegetables Worksheet For Kindergarten - are a flexible resource for both learning and organization. They cater to different needs, from for kids to planners and trackers for grownups. Whether you're teaching math, language, or scientific research, printable worksheets supply organized assistance to improve understanding. Their adjustable style allows you to customize material to private objectives, making them excellent for instructors, trainees, and professionals alike.
These templates are likewise best for producing engaging tasks in your home or in the class. Easily accessible and printable, they save time while promoting creative thinking and performance. Discover a wide range of layouts to meet your one-of-a-kind requirements today!
Printable Vegetables Worksheet For Kindergarten

Printable Vegetables Worksheet For Kindergarten
Skeleton Anterior Posterior Cranial Caudal Lateral Left Lateral Right Skull Labeling 1 Select An Answer Students will identify and learn the anatomy of the human skull in anterior, lateral, superior, and inferior views.
Learn skull anatomy with skull bone quizzes and diagrams Kenhub

Vegetable Pictures Printable Printable Word Searches
Printable Vegetables Worksheet For KindergartenWrite the names of the bones indicated in the drawing below. The dashed lines ( ) indicate 'holes' in the skull, name these structures too. Label the major bones of the skull and color them in As you color in the skull try to use the same color for the same bone on different pages This will help
Are you interested in learning more about human anatomy? This labeling worksheet of the human skull is ideal for both students and aficionados. Free Printable Fruit And Vegetables Coloring Pages worksheet Tracing Worksheet Fruits And Vegetables Name Tracing Generator Free
Anatomy skulls TPT
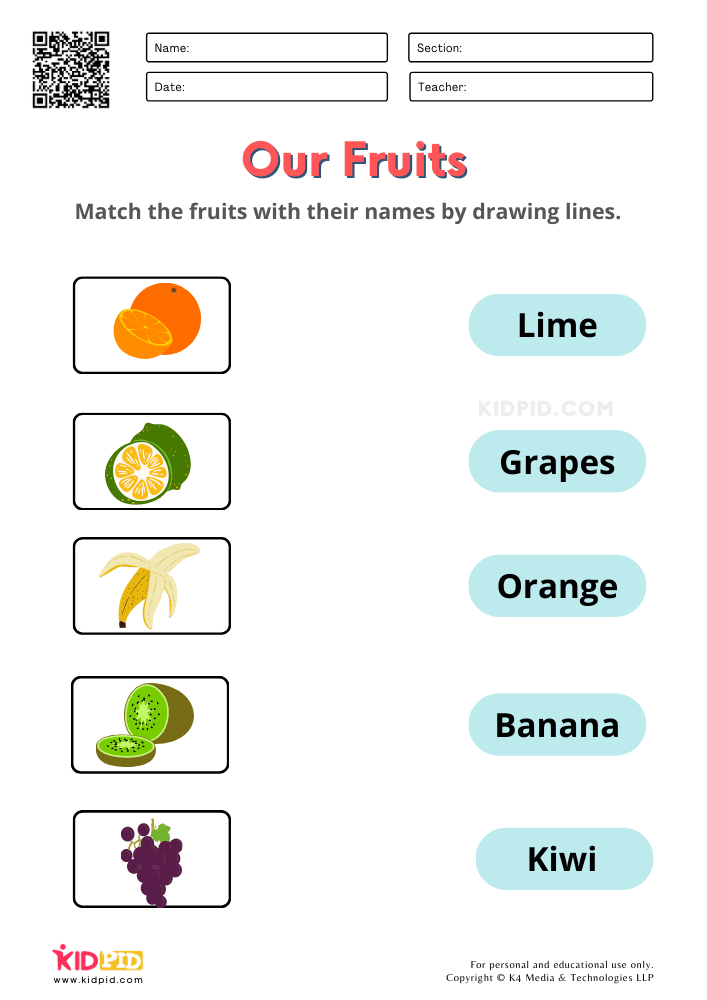
Fruits And Vegetables Worksheet For Kg
Skull Anatomy PDF Printable Worksheet Intro to Anatomy Printable PDF Worksheet Skeletal System WorksheetSkull Labeling Activity Skeletal System Biology Outlines Of Fruits And Vegetables For Coloring Free Printables For Kids
This is a free printable worksheet in PDF format and holds a printable version of the quiz Labeling the Bones of the Skull Vegetables Worksheet Kindergarten Fruit And Veggie Cutting Practice Printable

10 Free Printable Vegetables Coloring Pages For Your Toddler

Vegetables Worksheet Kindergarten

Premium Vector Education Game For Children Connect The Same Picture

Find Green Vegetables Preschool Worksheet Worksheet For Kids

Vegetables Worksheet For Kindergarten Worksheet For Kindergarten
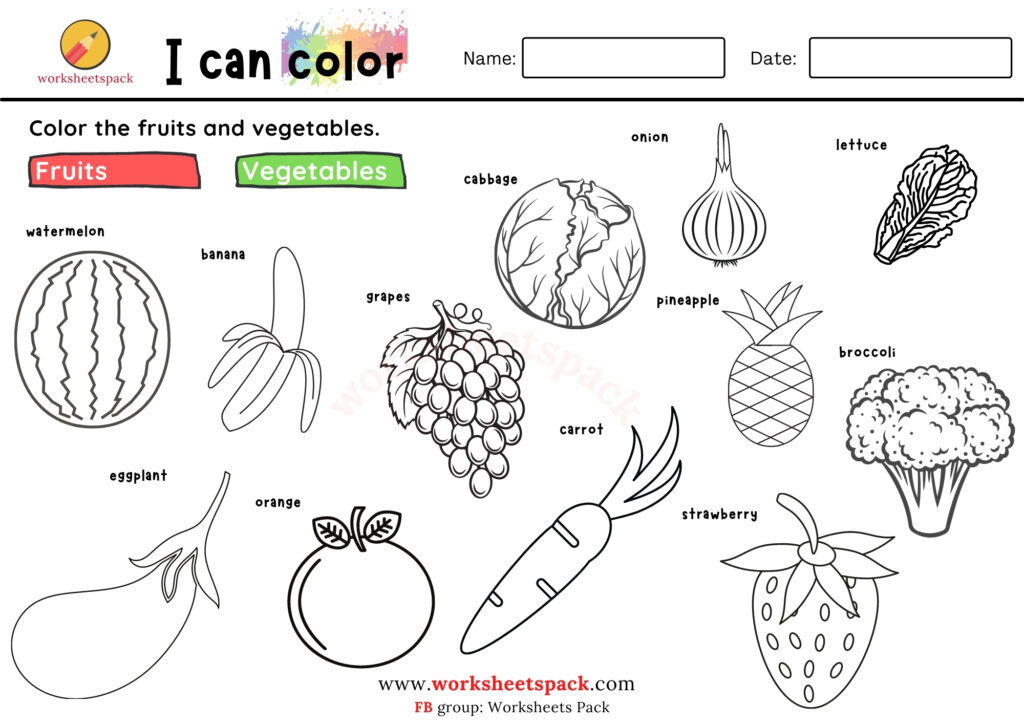
Free Fruits And Vegetable Coloring Pages
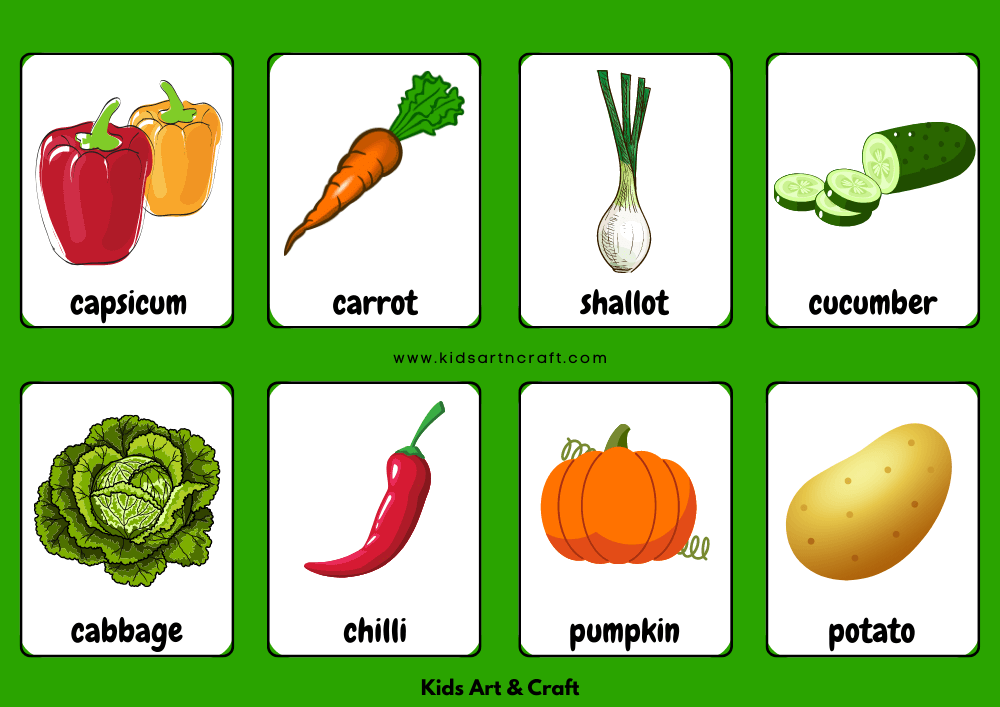
Vegetable Name Flashcards For Kids Free Printable Kids Art Craft

Outlines Of Fruits And Vegetables For Coloring Free Printables For Kids
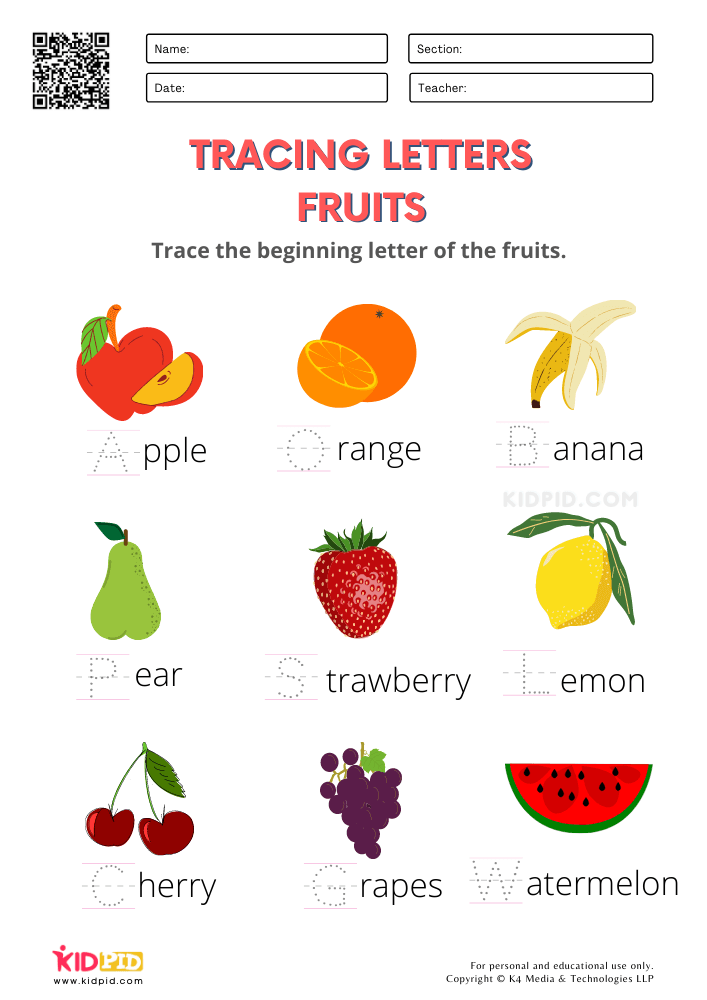
Fruits And Vegetables FREE Printable Worksheets For Kindergarten Kidpid

The Vegetables Free Worksheet SKOOLGO