Free Printable Number Writing Worksheets For Kindergarten - are a flexible source for both understanding and company. They accommodate numerous needs, from for kids to planners and trackers for grownups. Whether you're instructing mathematics, language, or science, printable worksheets give structured guidance to improve understanding. Their personalized style permits you to tailor content to individual objectives, making them excellent for instructors, students, and professionals alike.
These templates are likewise best for creating interesting activities in the house or in the class. Conveniently accessible and printable, they save time while advertising creativity and performance. Discover a variety of designs to fulfill your unique requirements today!
Free Printable Number Writing Worksheets For Kindergarten

Free Printable Number Writing Worksheets For Kindergarten
Grab these free printable music worksheets for elementary age kids to learn the musical symbols instruments and introduction theory Check out our variety of music themed worksheets for kids that will help them learn about musical instruments and practice some important skills like matching, counting, spelling and more! Find many printable music worksheets at AllKidsNetwork.
Free Music Theory Worksheets MakingMusicFun
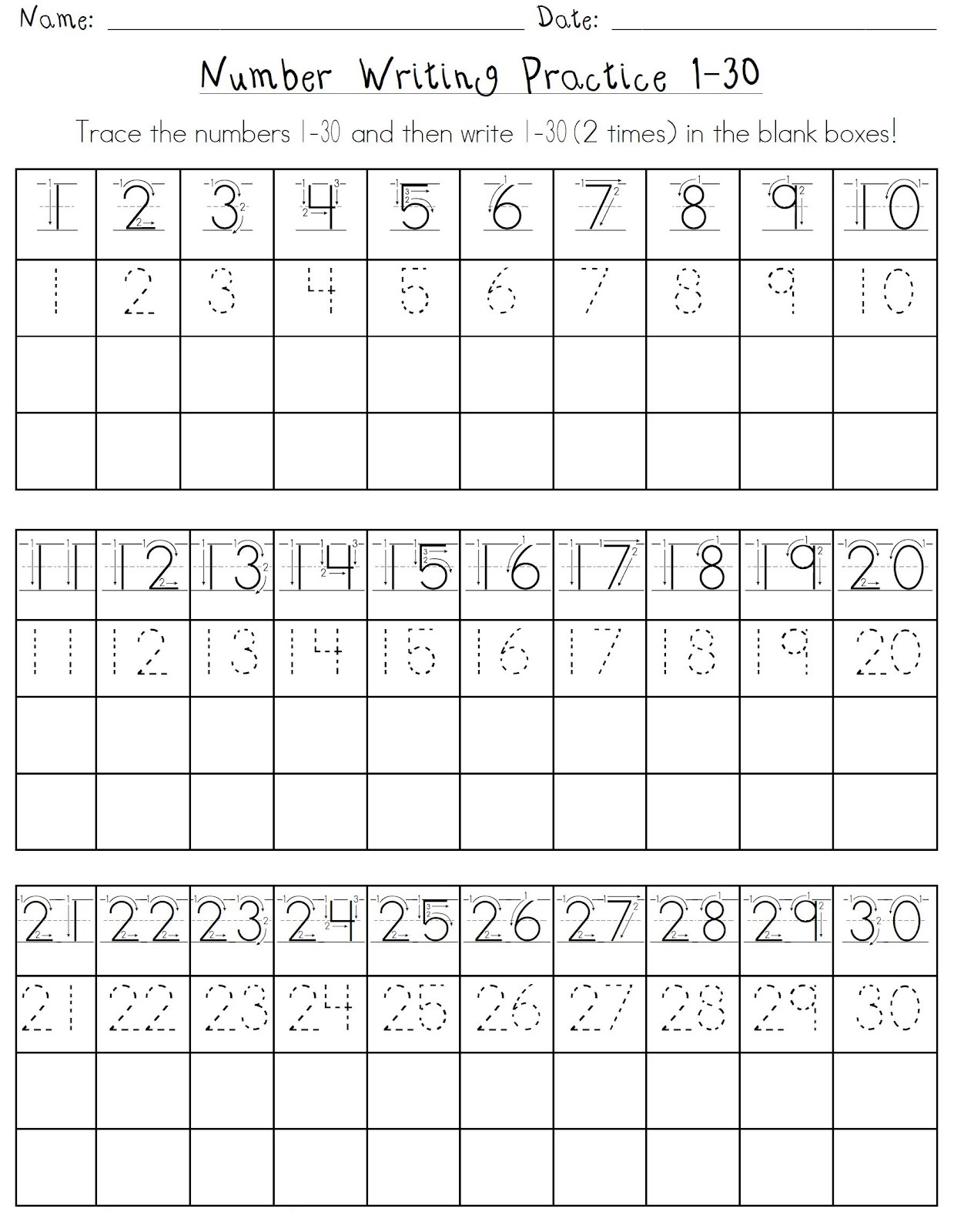
Number Line Practice Sheets
Free Printable Number Writing Worksheets For KindergartenFree sheet music of traditional nursery rhymes and children's songs and free fun and easy music theory printable worksheets for kids. Enjoy these free music worksheets and enrich your teaching or personal learning journey Whether you re working on note identification or perfecting drawing clefs these worksheets will give you support as you study and practice Happy playing
· Here is a fun worksheet that will walk you through analyzing a music video in writing! Learn about the rules for using commas with these free, printable magical musical posters. Practice your typing skills while playing songs with this GarageBand typing activity! Preschool Worksheets To Print Out Preschool Worksheets Number Activities For Kindergarten
Printable Music Worksheets For Kids All Kids Network

Free Printable Number Writing Practice
Nurture musical learning with worksheets for kids Designed for Pre K to elementary levels these resources make music theory fun engaging Number 5 Worksheets For Kindergarten
Children s Songs free printable sheet music with lyrics PDF This collection of printable Sheet Music PDF files for free download includes simple worksheets for nursery rhymes lullabies and folk songs as well as preschool music worksheets Numbers From 1 To 20 Worksheets Free Tracing And Writing Number 5 Worksheet Kids Activities
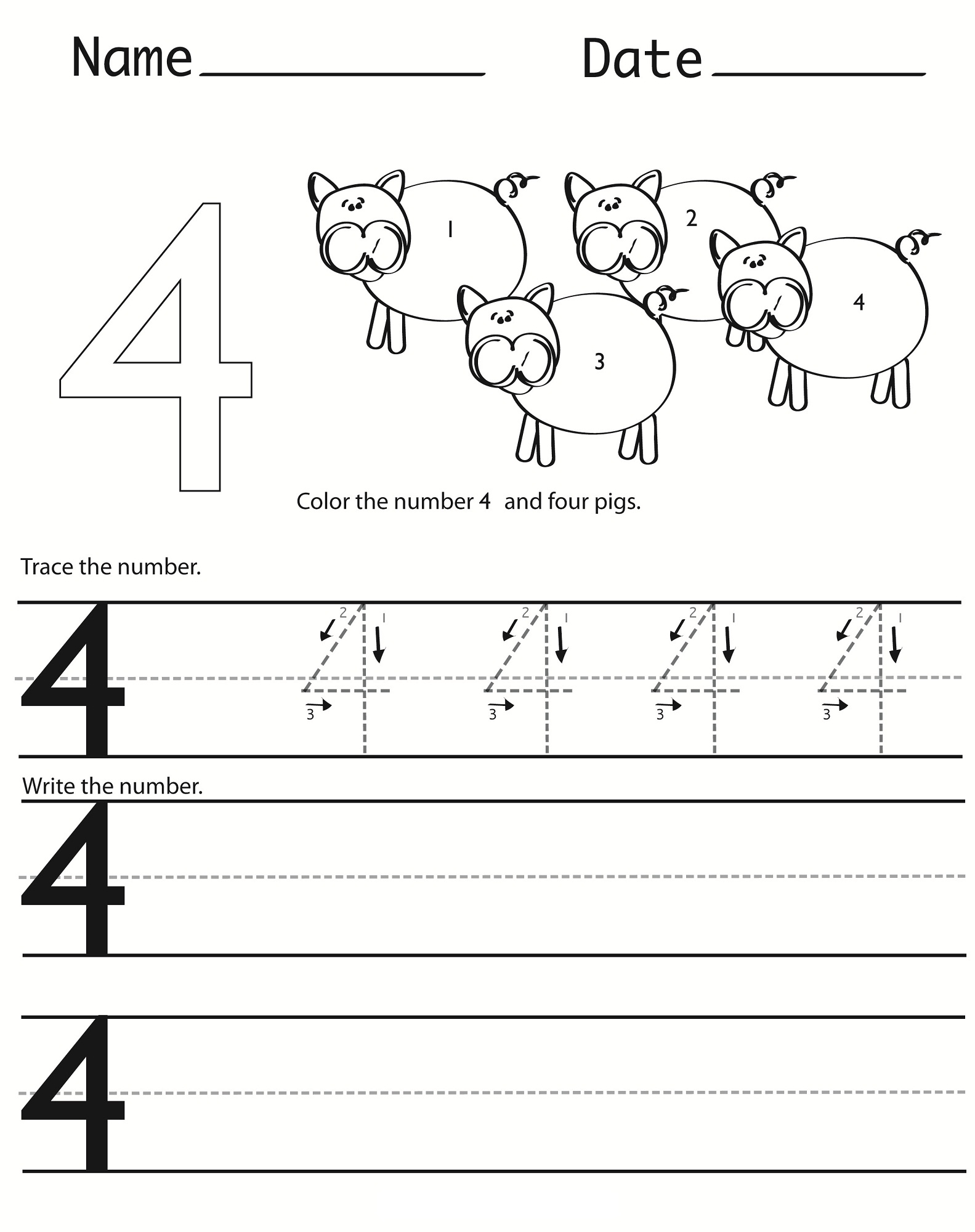
Number 4 Worksheets For Children Activity Shelter

Grade Level Worksheets A Wellspring Of Worksheets Writing Numbers

Free Printable Number Word Worksheets

Numbers Trace And Write
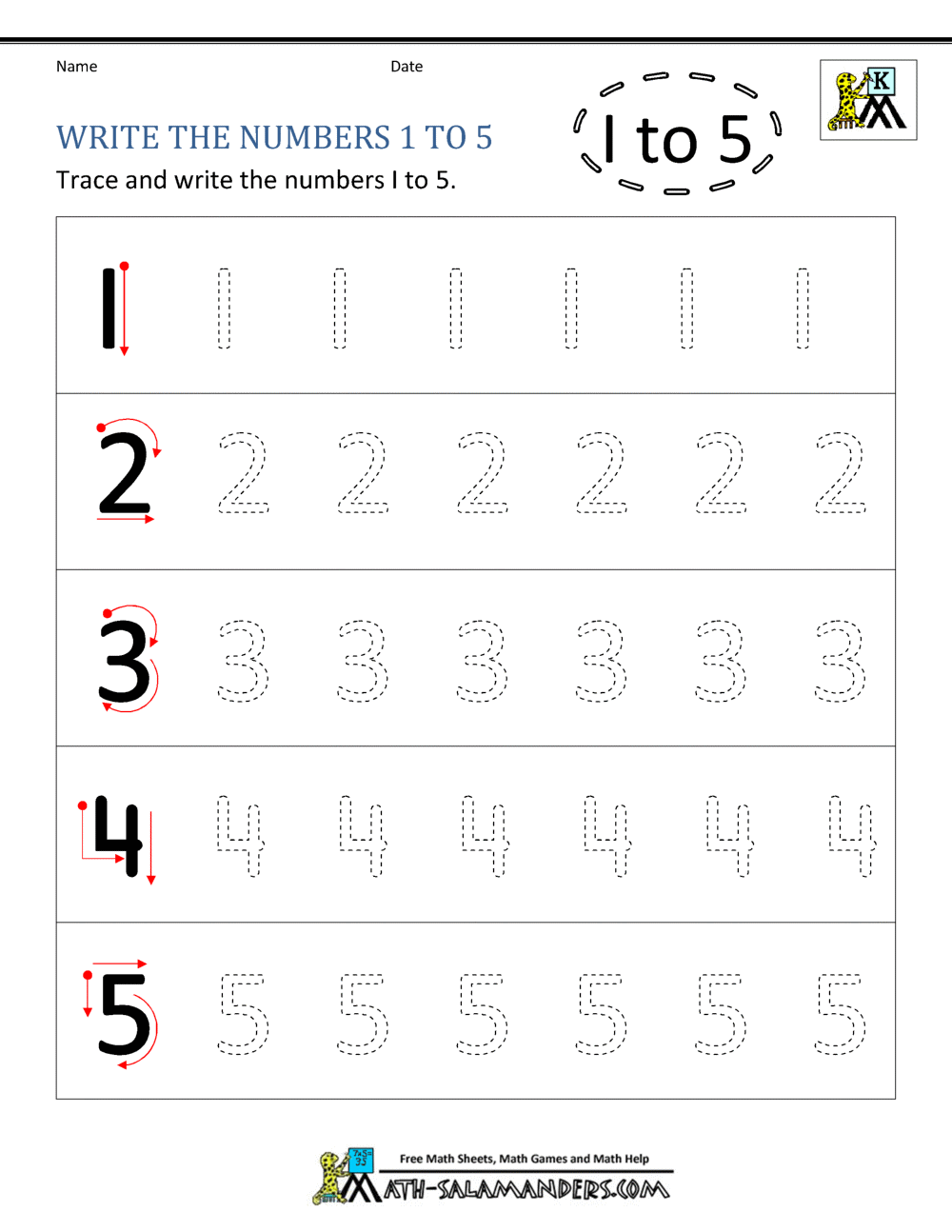
Kindergarten Printable Worksheets Writing Numbers To 10

Writing Numbers Worksheets Printable Activity Shelter
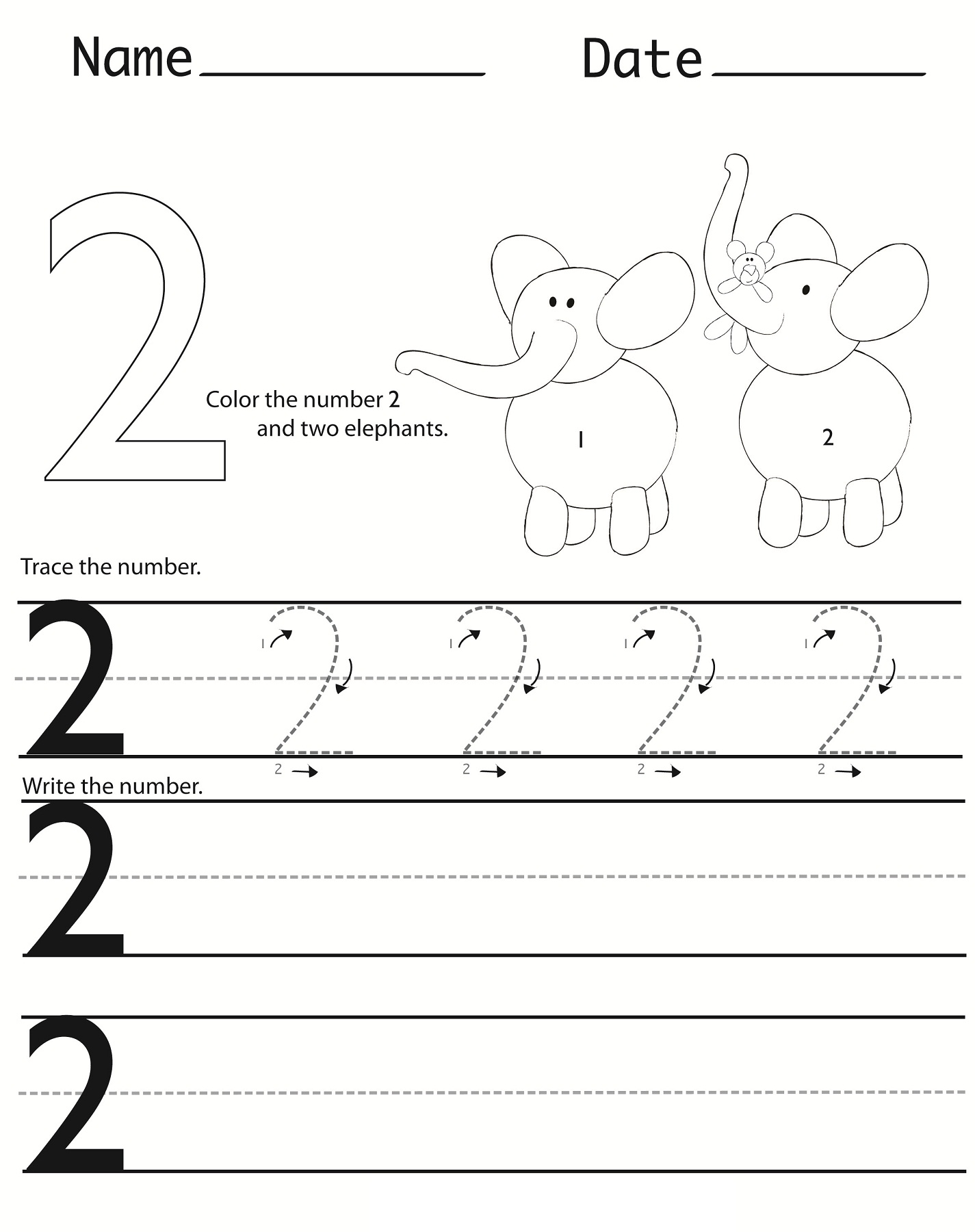
Writing Numbers Worksheet Printable
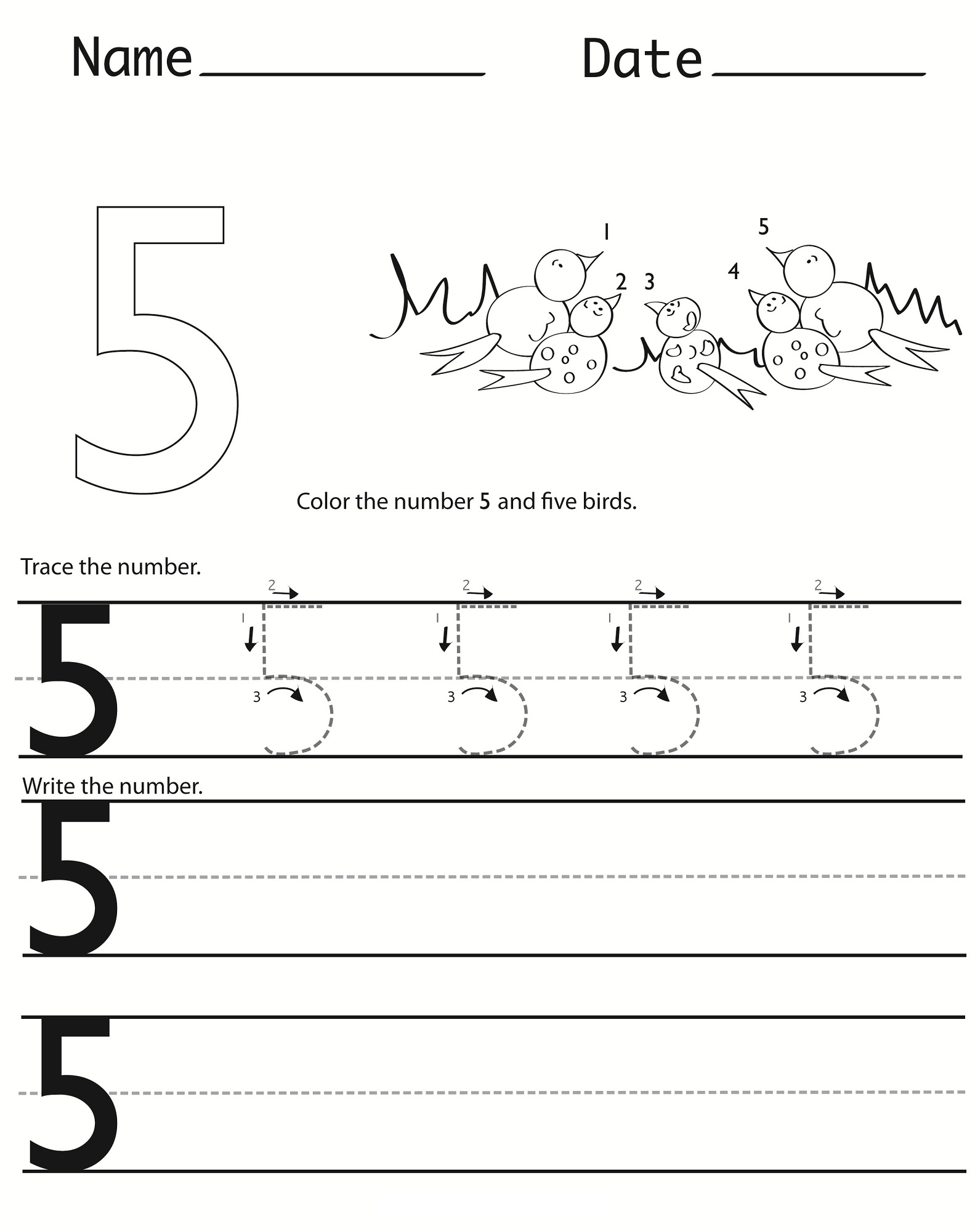
Number 5 Worksheets For Kindergarten
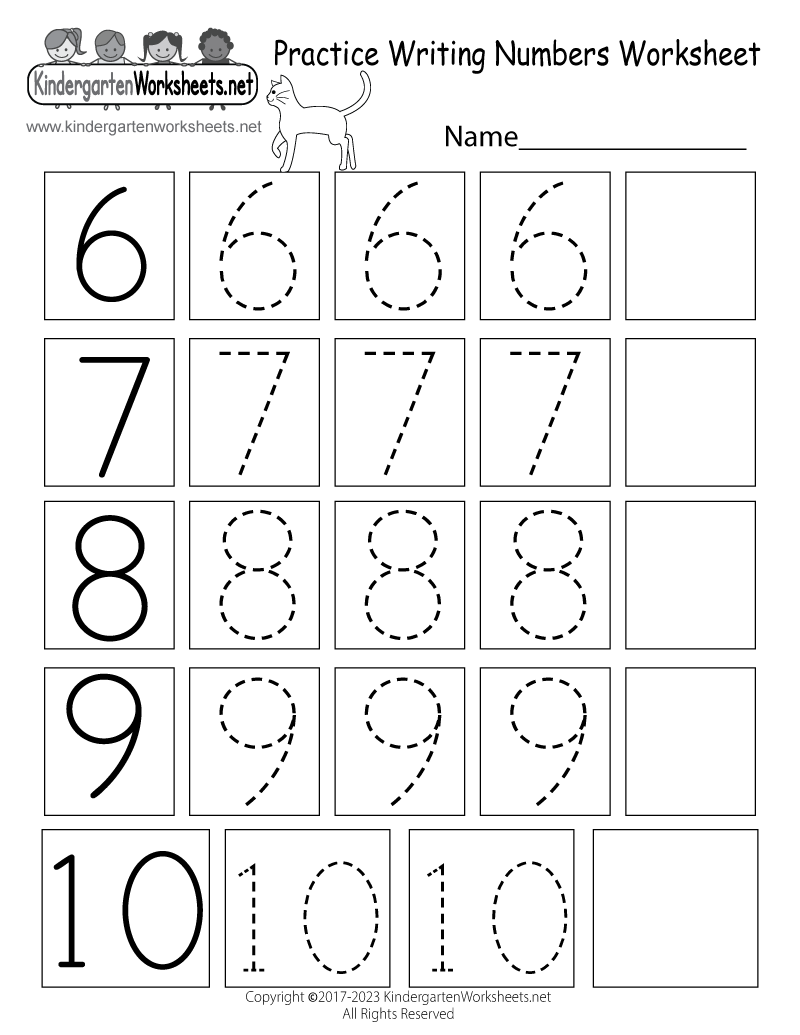
Writing Numbers Activities For Kindergarten
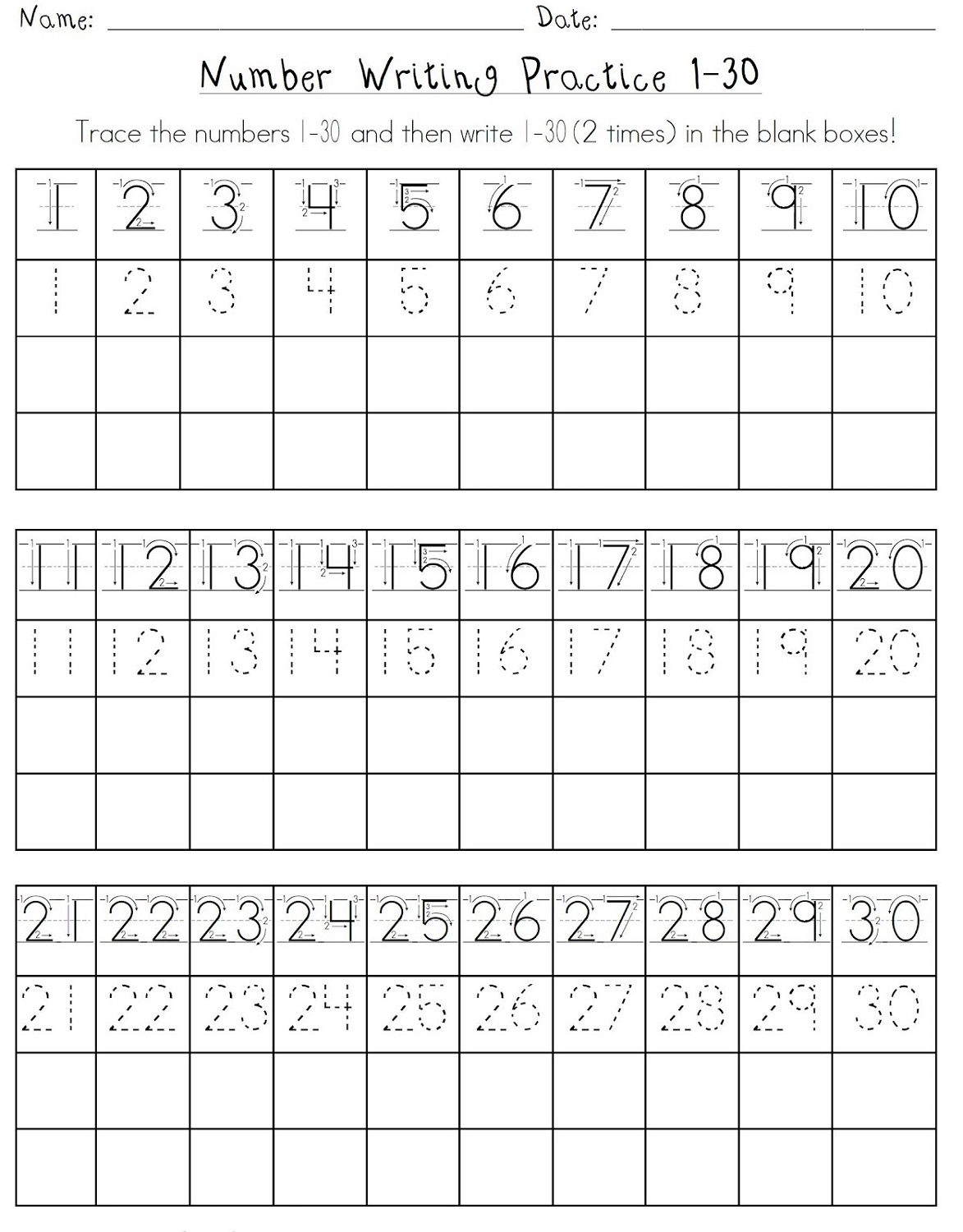
Printable Trace Sheets