Free Printable Making 10 Worksheets - are a functional resource for both knowing and organization. They cater to different needs, from educational activities for children to planners and trackers for grownups. Whether you're educating mathematics, language, or scientific research, printable worksheets offer structured assistance to improve understanding. Their personalized format permits you to customize content to individual goals, making them suitable for instructors, students, and specialists alike.
These templates are also best for producing appealing tasks in your home or in the classroom. Easily available and printable, they save time while advertising imagination and productivity. Check out a wide variety of styles to fulfill your unique needs today!
Free Printable Making 10 Worksheets
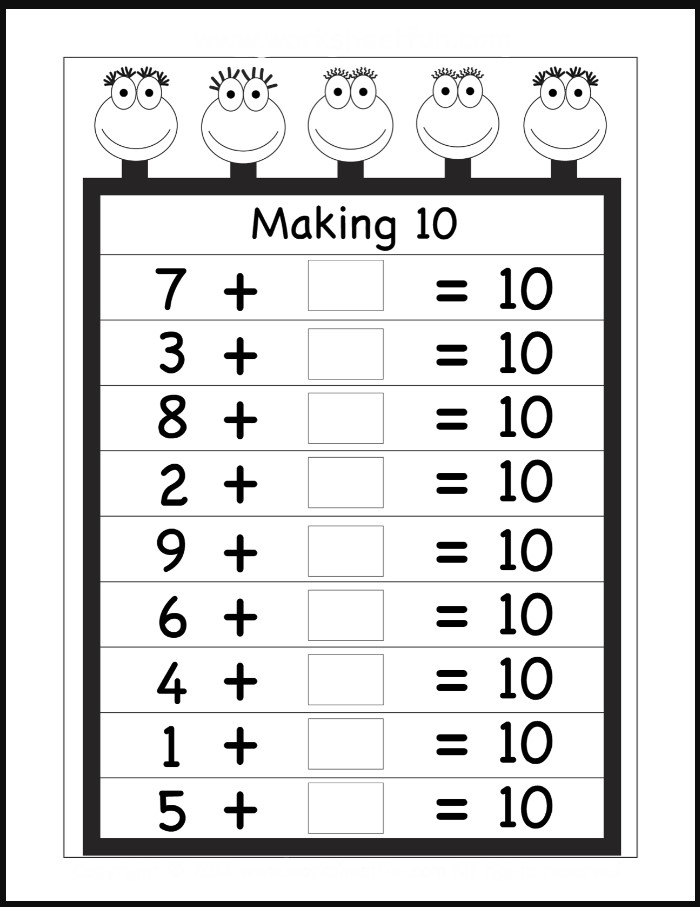
Free Printable Making 10 Worksheets
Average Atomic Mass Overview The average atomic mass of an element can be determined from the relative amounts of each isotope This is the mass used in most chemical calculations In a naturally occurring element the fractional abundance is the percentage of the abundance of a particular isotope in the total sample of atoms written as a decimal Calculate the average atomic mass of lithium, which occurs as two isotopes that have the following atomic masses and abundances in nature: 6.017 u, 7.30% and 7.018 u, 92.70%.
Isotopic Abundance Practice Problems Maurer Math

Free Making Ten Worksheet Kindergarten Worksheets Math Worksheets
Free Printable Making 10 WorksheetsThe average atomic mass of an element is the reason atomic mass is not a whole number in the Periodic Table. It is a weighted average of the all masses of all the isotopes that exist for that element. It can easily be calculated by the formula shown below. Average atomic mass = (% abundance)(mass of isotope) +(% abundance)(mass of isotope).….. Calculate the average atomic mass for each element based on the natural abundance of its isotopes Find the average atomic mass for Li if 7 5 of Li atoms are 6Li with a mass of 6 0151223 amu and 92 5 are 7Li with a mass of 7 0160041 amu
[desc_9] [img_title-17] [img_title-16]
More Average Atomic Mass S W H S CHEMISTRY

Addition Making 10 Worksheets Free Download Gambr co
[desc-8] [img_title-11]
Calculate the average atomic mass of Sulfur if 95 00 of all sulfur atoms have a mass of 31 972 amu 0 76 have a mass of 32 971amu and 4 22 have a mass of 33 967amu The four isotopes of Lead are shown below each with its percent by mass abundance and the composition of [img_title-12] [img_title-13]

Make 10 Worksheet Printable Word Searches

Let s Make Ten With Ten Frames Roll A Die Complete A Number Sentence
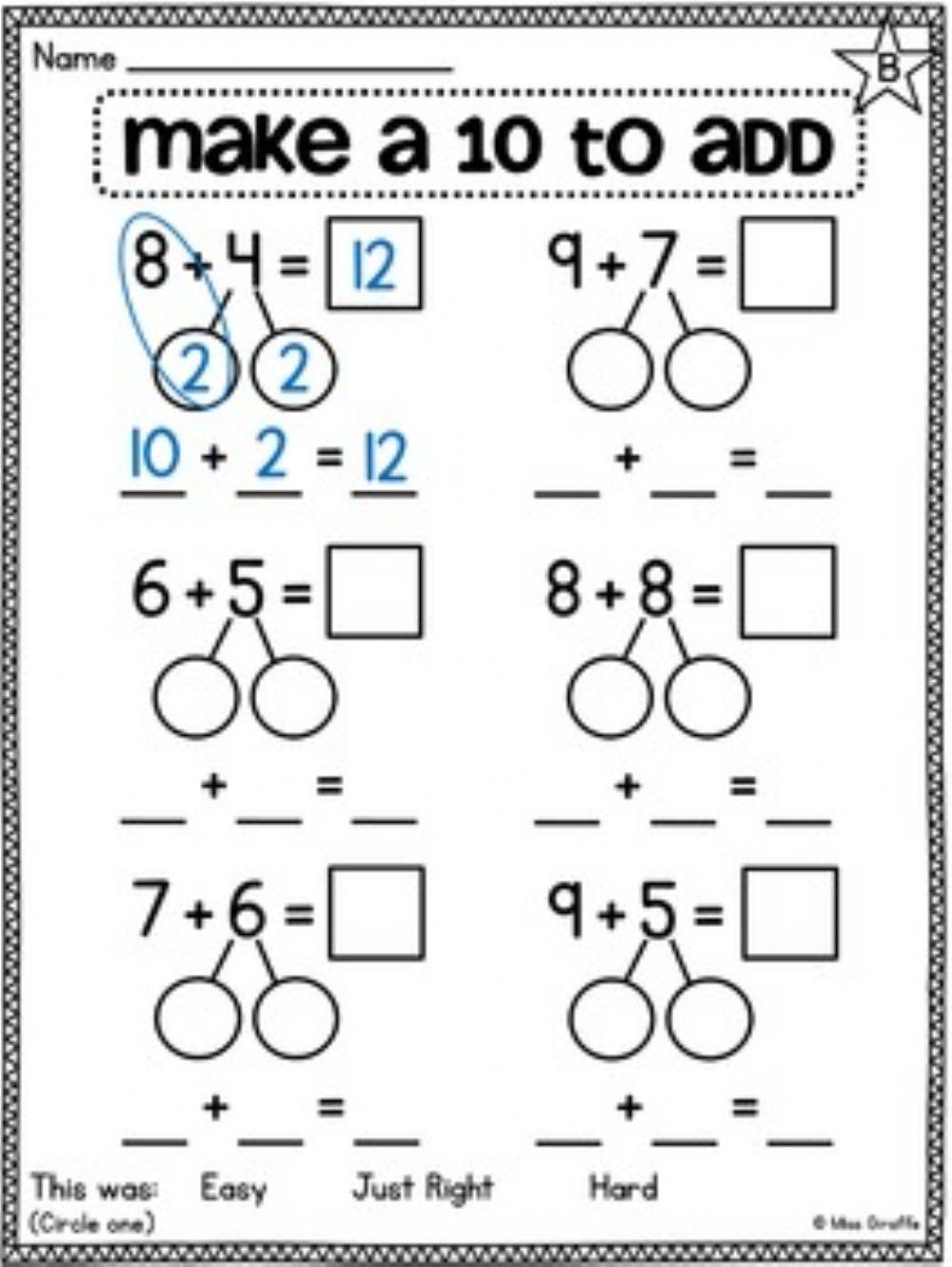
Make Ten Addition Strategy A Worksheets Library
[img_title-7]
[img_title-8]
[img_title-9]
[img_title-10]
[img_title-11]
[img_title-14]
[img_title-15]