First Grade Printable Worksheets - are a functional source for both discovering and organization. They deal with different demands, from for youngsters to planners and trackers for grownups. Whether you're showing math, language, or science, printable worksheets provide structured support to boost understanding. Their adjustable style enables you to customize content to private objectives, making them perfect for instructors, pupils, and experts alike.
These templates are also best for producing engaging activities in your home or in the class. Quickly obtainable and printable, they save time while promoting creativity and efficiency. Discover a vast array of layouts to fulfill your one-of-a-kind needs today!
First Grade Printable Worksheets

First Grade Printable Worksheets
Hess s Law Worksheet answers 1 Calculate H for the reaction C2H4 g H2 g C2H6 g from the following data C2H4 g 3 O2 g 2 CO2 g Given the following data: C2H2(g). + 2.5 O2(g) → 2 CO2(g). + H2O(l). ∆H = - 1300 kJ. C(s) + O2(g) → CO2(g). ∆H = -394 kJ. H2(g). + ½ O2(g) → H2O(l).
Answers to Hess s Law Worksheet

Pin By Hand Made By Nature On Table Setting And Presentation First
First Grade Printable WorksheetsThe standard enthalpy for the combustion of hexane is −1560 kJ/mol. Multiplying −1560 by 2 and then adding +264 will yield −2856. 1 Find the AH for the reaction below given the following reactions and subsequent AH values PCls g PC13 g Cl2 g
Hess' Law Worksheet. Using your knowledge of Hess' law, answer the following questions: 1). Calculate ΔH for the reaction 3 Cl2(g) + CS2(l) → CCl4(l) + S2Cl2 ... Reading Exercises For First Graders Counting coins worksheets Tim s Printables
Hess s Law Problems

1st Grade Money Worksheets Math Monks
Answers to Hess s Law Worksheet Problem 1 Calculate the enthalpy for the following reaction N 2 g 2O 2 g 2NO 2 g H kJ Using the following two 10 Printable Two Digit Addition Worksheets Double Digit Addition
1 Find the AH for the reaction below given the following reactions and subsequent AH values 2 CO2 g H2O g C2H2 g O2 g Subtraction 3rd Grade Math Worksheets 3rd Grade Math Worksheets Alphabet Worksheets K5 AlphabetWorksheetsFree
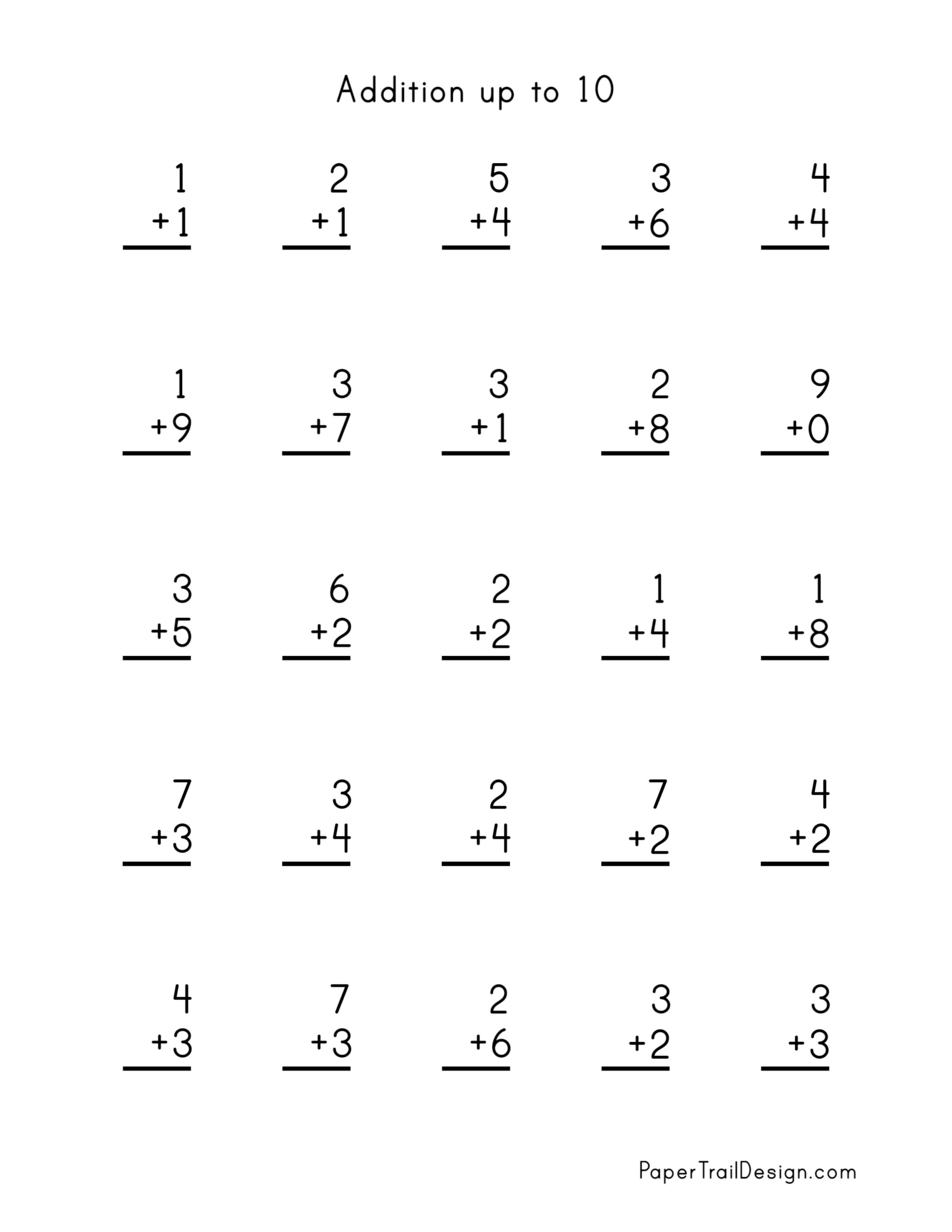
Addition Worksheets Paper Trail Design

Multiplication Worksheets One Digit Math Drills DIY Projects
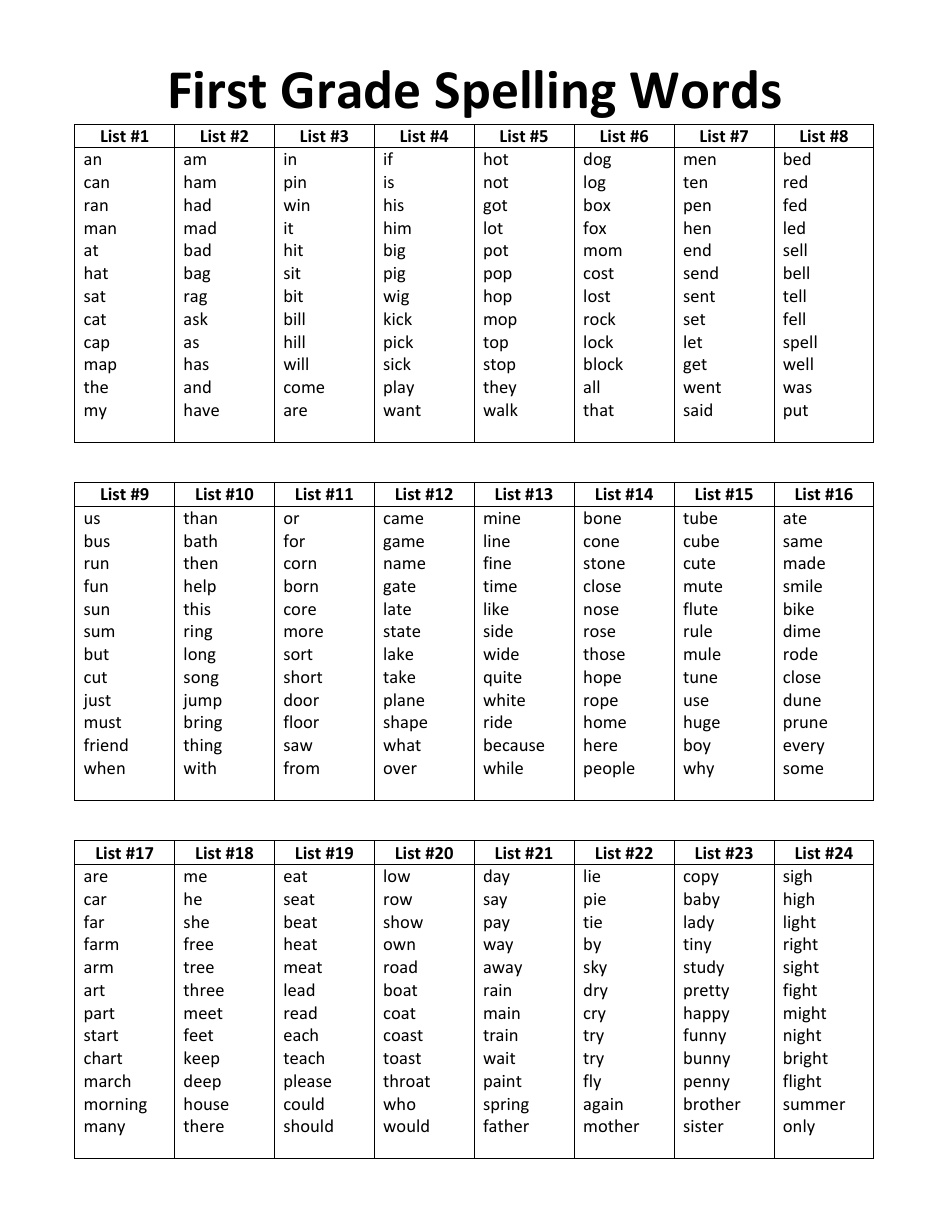
Hard Spelling Bee Words

Subtraction 1st Grade Math Worksheets Printable Activity Book

Learn The Number 1 Free Worksheet For Kids SKOOLGO
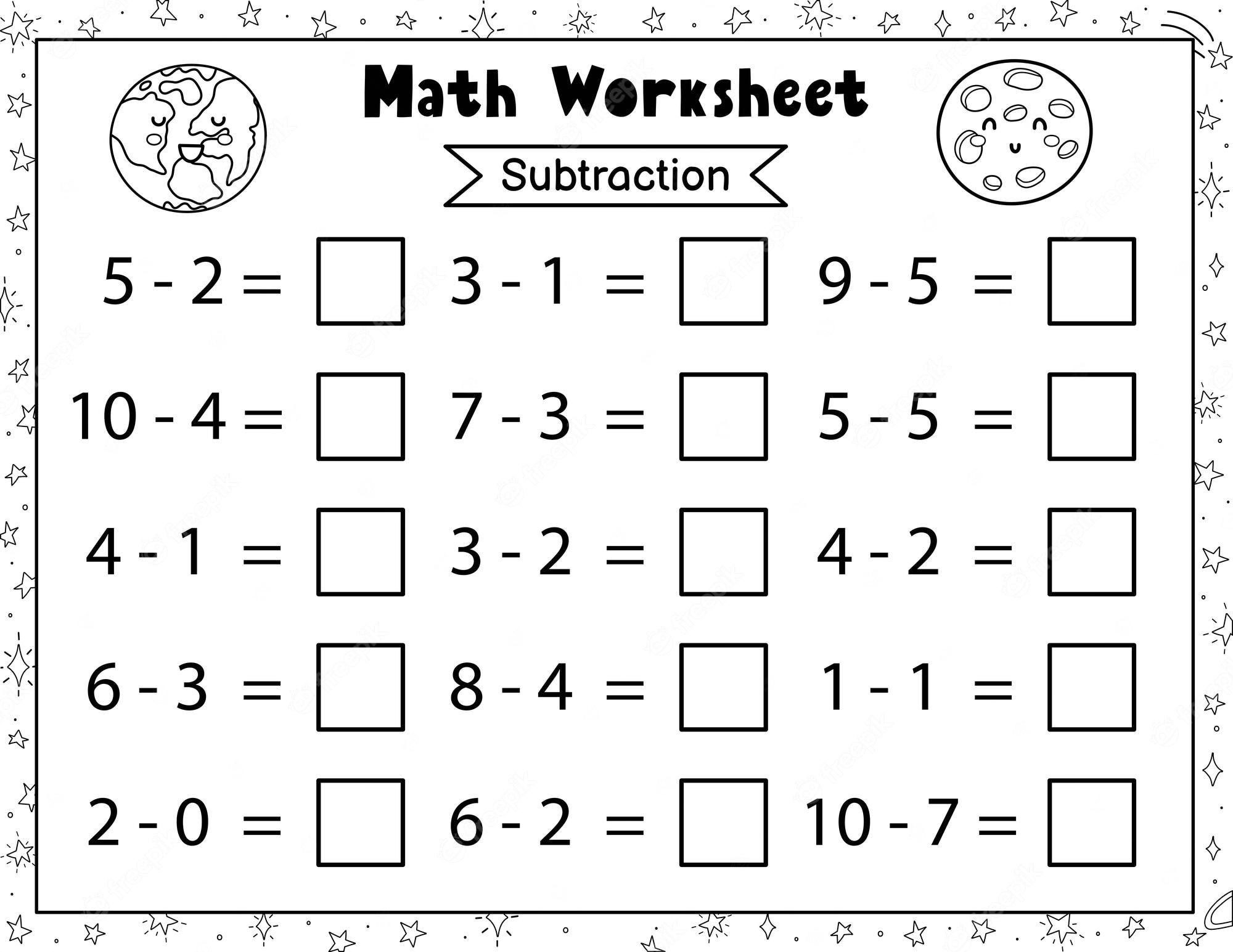
1st Grade Math Worksheets Engaging And Educational Activities

FREE St Patrick s Day 1st Grade Worksheets And Activities No Prep

10 Printable Two Digit Addition Worksheets Double Digit Addition

Addition And Subtraction Worksheets Learning Printable

Missing Addend Worksheet First Grade