Esl Printable Worksheets - are a versatile resource for both knowing and company. They satisfy different requirements, from for kids to planners and trackers for adults. Whether you're educating mathematics, language, or science, printable worksheets supply structured guidance to improve understanding. Their customizable style allows you to customize web content to individual goals, making them excellent for educators, students, and experts alike.
These templates are also perfect for producing interesting tasks at home or in the class. Conveniently accessible and printable, they save time while advertising imagination and productivity. Discover a variety of layouts to fulfill your one-of-a-kind demands today!
Esl Printable Worksheets
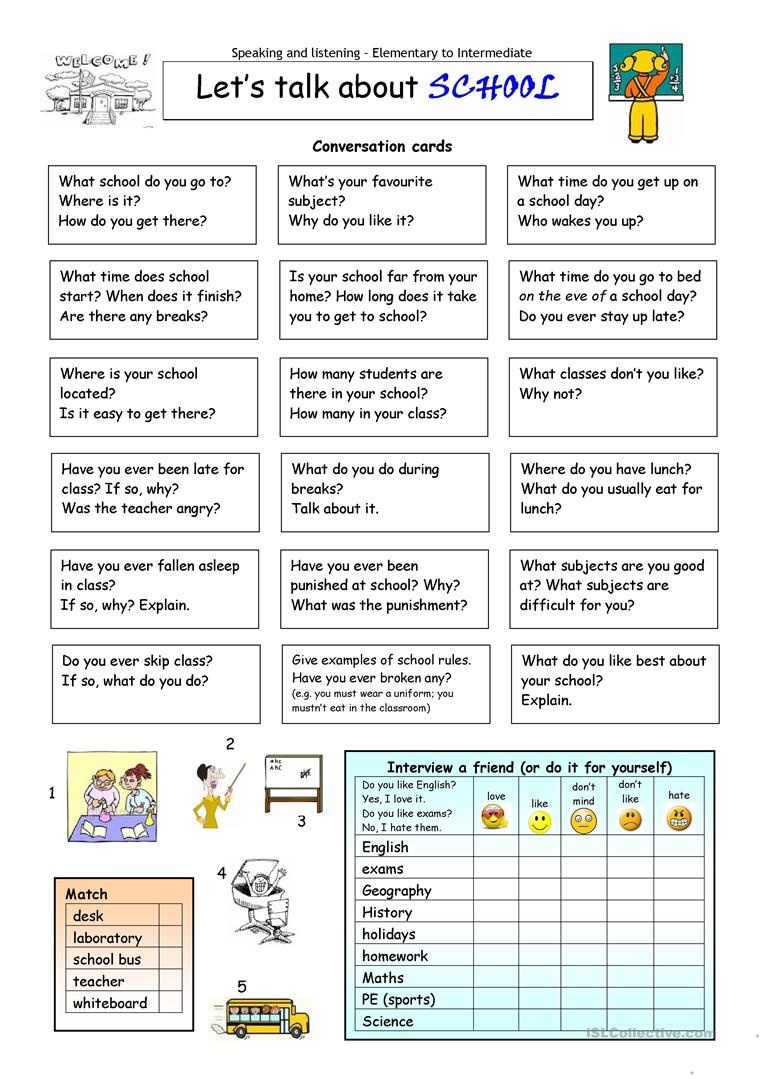
Esl Printable Worksheets
Free grade 5 measuring worksheets Create an unlimited supply of worksheets for conversion of measurement units for grade 5 both customary and metric units On this webpage, you will find a range of measurement worksheets to help your child learn to read scales and work with both standard and metric units.
5th grade measurement conversions worksheets TPT

Free Printable Esl Worksheets For Beginners
Esl Printable WorksheetsStudents convert length measurements within the metric system in this helpful practice worksheet! 5th grade. Math. Interactive Worksheet. This collection of worksheets is made to measure for fifth grade math students Our fifth grade measurement worksheets provide practice with calculating solid
These measurement worksheets are a great resource for teachers, students, and parents looking to improve their measurement skills. Try them out today and see ... ESL Printable Worksheets For Kids Worksheets Library Group 1 Sports Read And Write Worksheet For K5 Kids And ESL Students
5th Grade Measurement Worksheets Math Salamanders
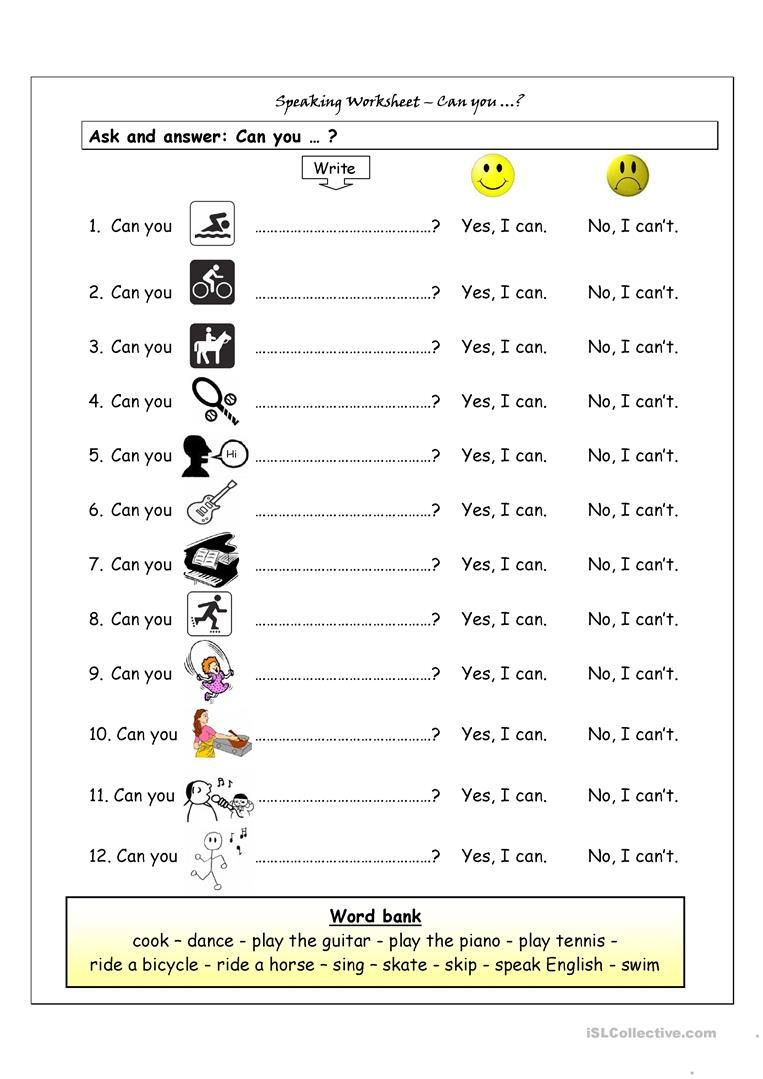
Free Esl Printable Worksheets
Measurement Conversion Worksheets are a valuable tool for 5th grade students who are learning to convert units of measurement Grammar For Beginners To Be Worksheet Free Esl Printable Free
Measurement worksheets beginning with size comparisons and progressing to measuring length weight capacity and temperature in customary and metric units Printable Esl Worksheets Fun Activity Worksheets For Adults Printable Printable Worksheets

All About Shapes Printable Preschool Worksheets Worksheets Library
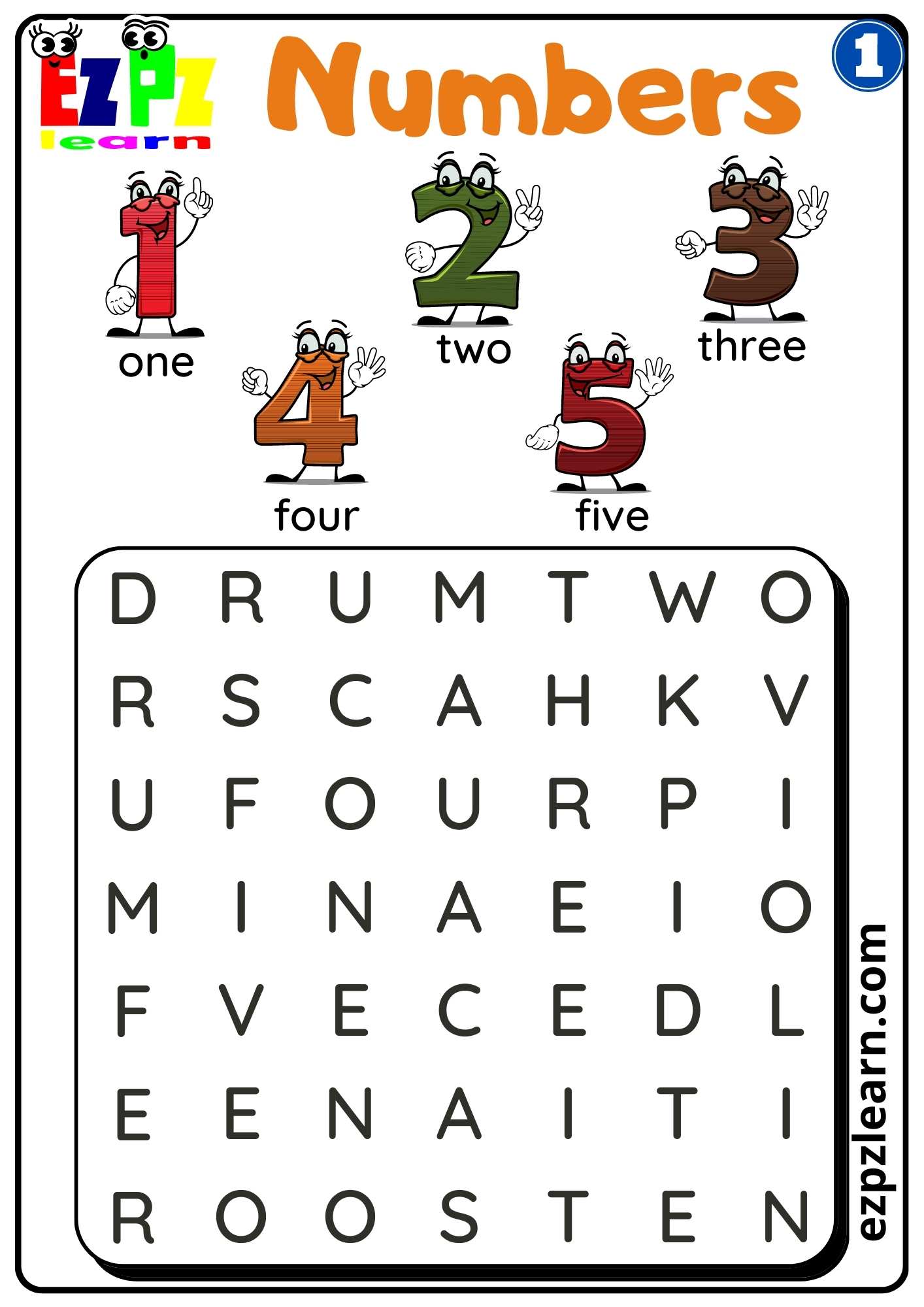
ESL Worksheets Free Printable Worksheets For ESL Vocabulary And

Finding The Theme Worksheets 99Worksheets Worksheets Library

Free Esl Printables For Adults Free Printable

Free Printable Esl Worksheets For Beginners
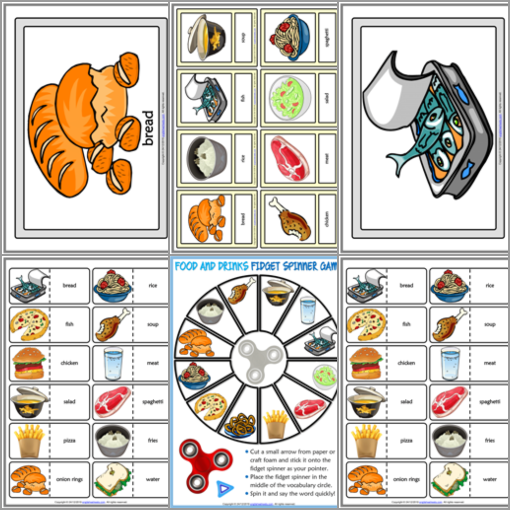
Free ESL Worksheets English Teaching Materials ESL Lesson Plans

Adult Cognitive Worksheets 14 Free PDF Printables Printablee

Grammar For Beginners To Be Worksheet Free Esl Printable Free

Esl Printables Grammar Worksheets

Printable English ESL Board Game Template Worksheet Printable Board