2nd Grade Multiplication Worksheets Free Printable - are a versatile source for both understanding and company. They satisfy various requirements, from for youngsters to planners and trackers for adults. Whether you're teaching mathematics, language, or science, printable worksheets offer organized advice to improve understanding. Their adjustable style enables you to tailor web content to specific goals, making them suitable for instructors, trainees, and professionals alike.
These templates are also best for creating engaging tasks at home or in the class. Conveniently easily accessible and printable, they save time while promoting imagination and efficiency. Explore a large range of styles to meet your unique demands today!
2nd Grade Multiplication Worksheets Free Printable

2nd Grade Multiplication Worksheets Free Printable
Comparing Fractions and Decimals Worksheets This Greater Than Less Than Worksheet is great for testing children in their comparison of Fractions and Decimals These 2 FREE worksheets will help your students practice comparing fractions and decimals with 10ths and 100ths. The second worksheet is more advanced with ...
Comparing Fractions and Decimals Worksheets Free Online PDFs
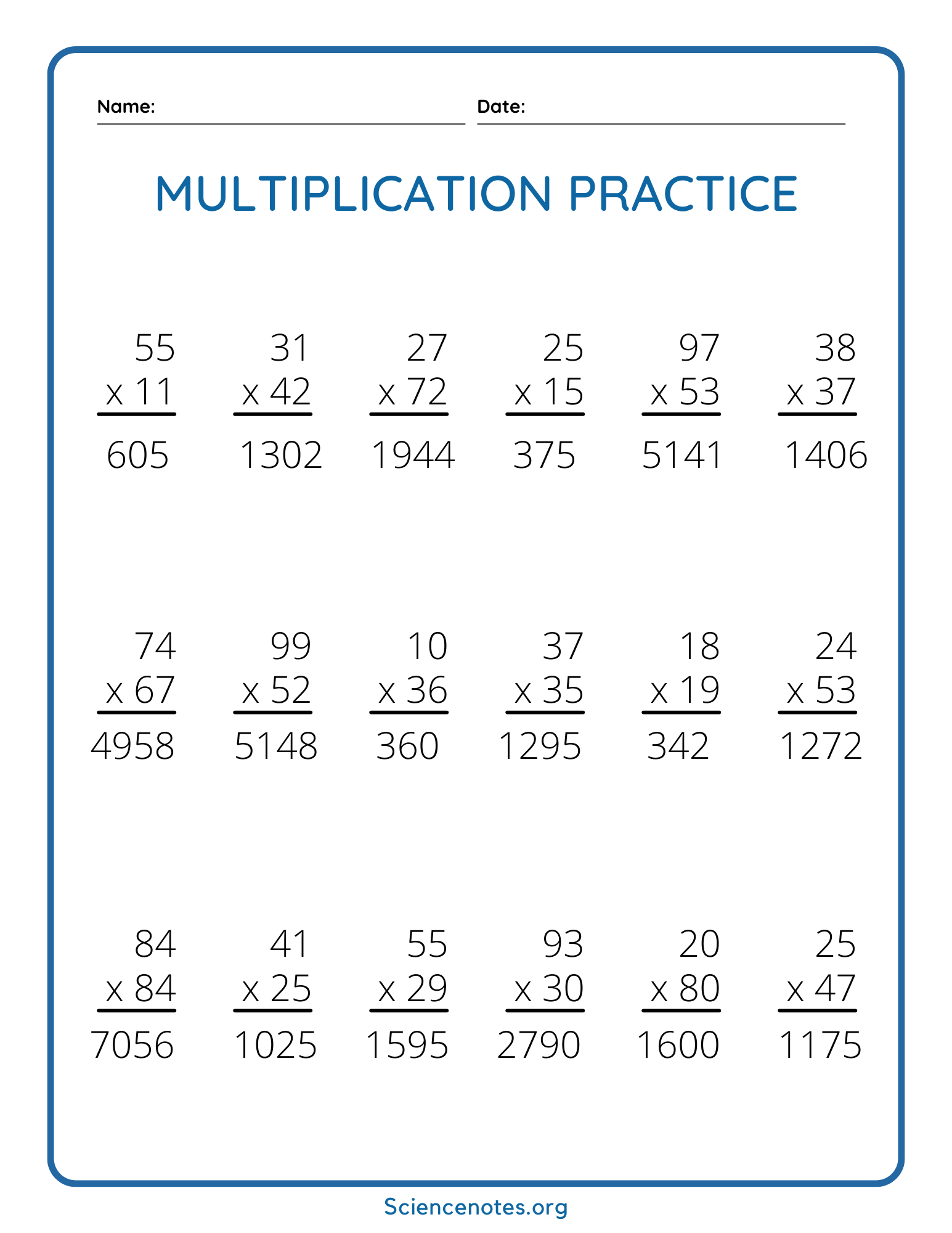
Free Multiplication Worksheets Printable Online Worksheets Library
2nd Grade Multiplication Worksheets Free PrintableIn this lesson, you will compare and order numbers. By ordering numbers, we are able to determine which numbers are bigger, smaller, the biggest, etc. These 2 FREE worksheets will help your students practice comparing fractions and decimals with 10ths and 100ths
Use this brilliant activity sheet to support your children's understanding of how to compare fractions and decimals. Division Worksheets Problems Free Printable Math Drills DIY Multiplication Worksheets For Grade 1 Math Monks
Compare Fractions and Decimals FREE Worksheets 4th and 5th

Beginning Multiplication Worksheets
Our comparing decimals worksheets contain exercises to compare decimals using greater than less than or equal to symbols and number lines Multiplication Worksheets 1 12 Math Fact Worksheets Multiplication
Free 3rd grade fractions and decimals worksheets including writing and comparing fractions equivalent fractions simplifying fractions adding and Multiplication Sheets 4th Grade 3rd Grade Math Fact Fluency Superhero 3rd Grade Math Fact Fluency

Beginning Multiplication Worksheets With Pictures Free Printable

1st Grade Multiplication Worksheets
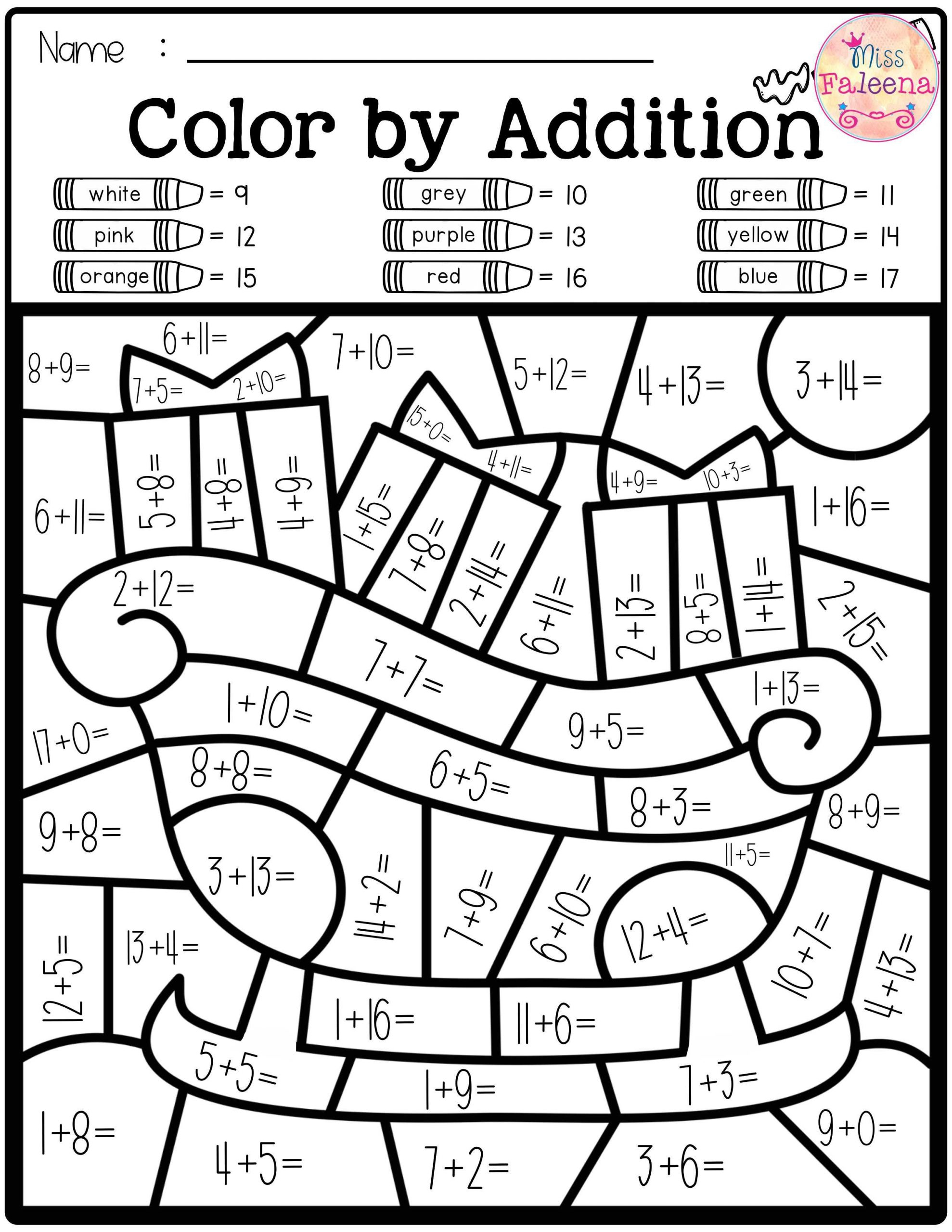
Free Printable Color By Number Worksheets For 3rd Grade Printable

Second Grade Multiplication Worksheets Teaching Multiplication
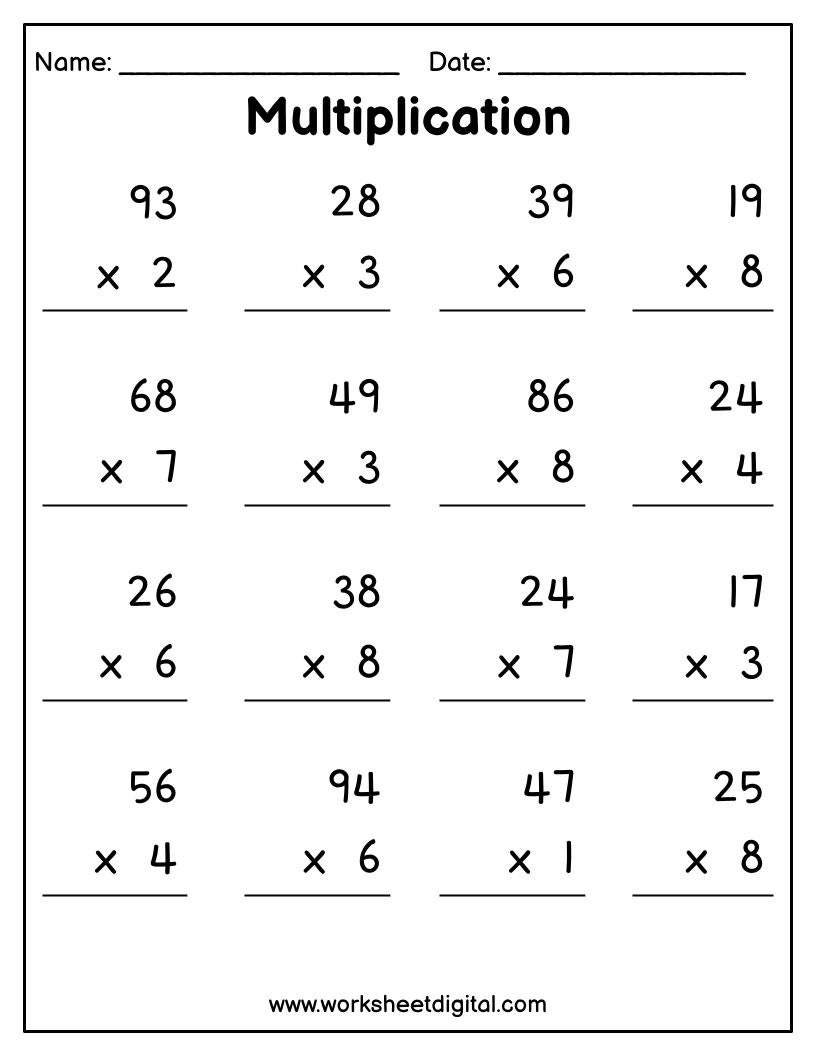
Free Multiplication Worksheets Multiplication Worksheets Library

Math Drills Multiplication Worksheet Worksheet
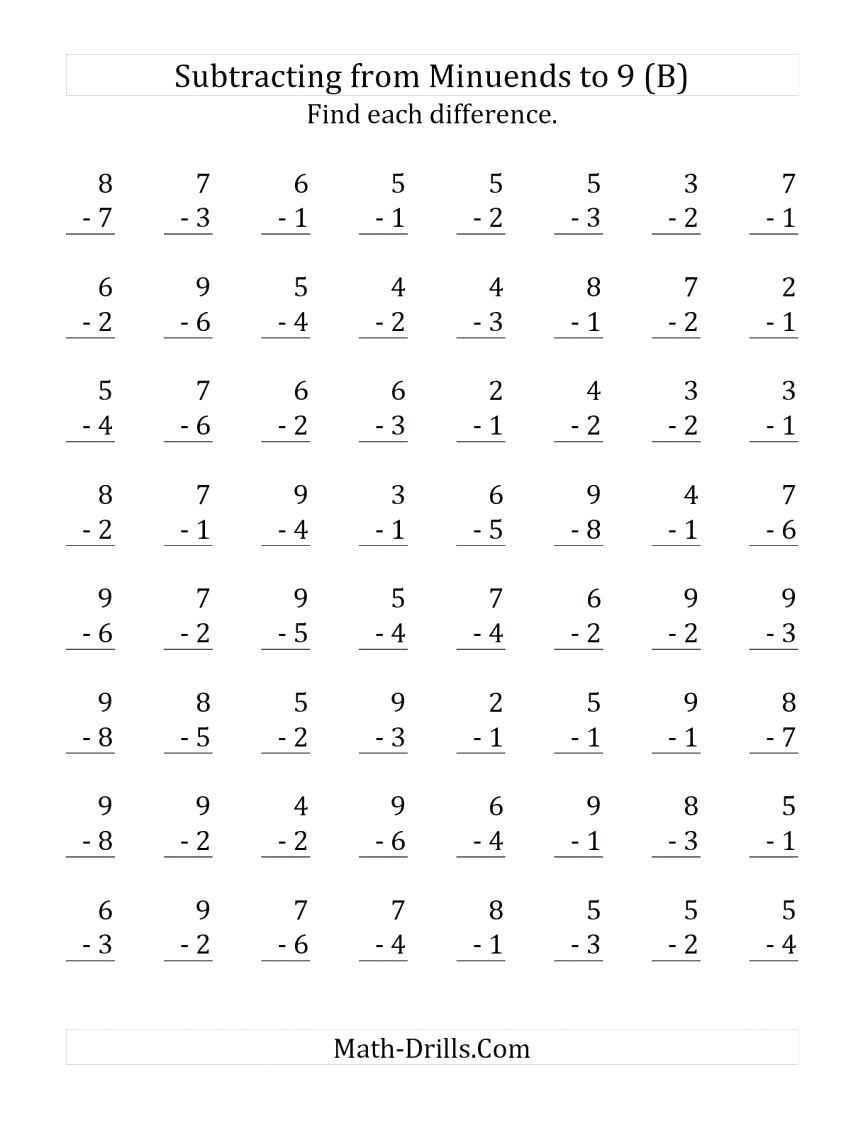
Second Grade Multiplication Worksheets Free Printable

Multiplication Worksheets 1 12 Math Fact Worksheets Multiplication
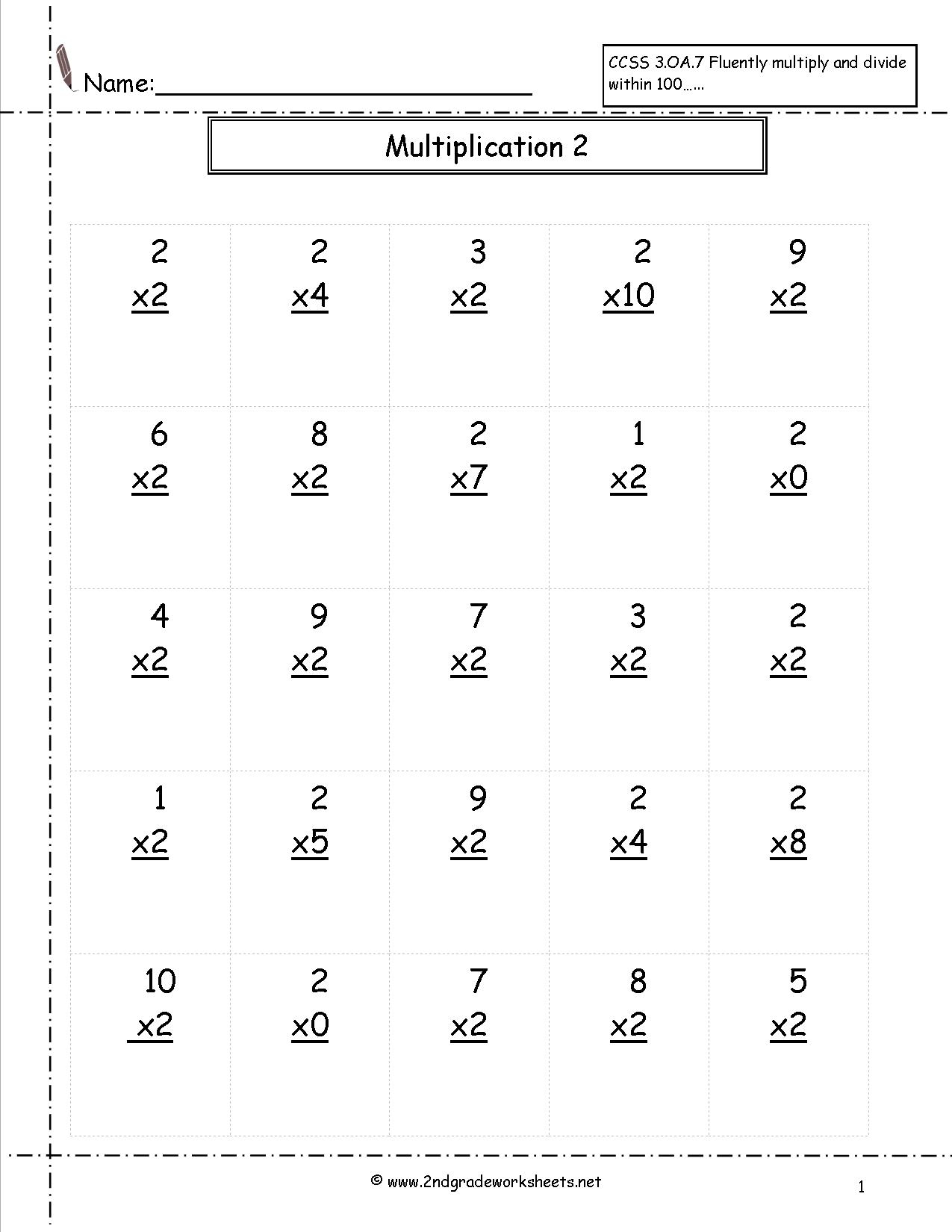
Second Grade Multiplication Worksheets Free Printable
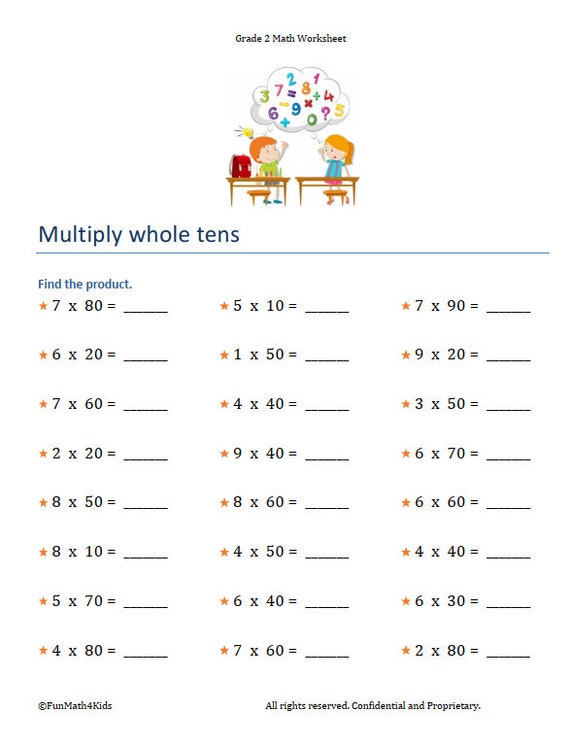
2nd Grade Math Worksheets Multiplication