Year 5 Spelling Worksheets Printable - are a functional source for both understanding and company. They satisfy different demands, from educational activities for kids to planners and trackers for grownups. Whether you're instructing math, language, or science, printable worksheets offer organized advice to boost understanding. Their adjustable format permits you to tailor web content to private goals, making them excellent for instructors, pupils, and specialists alike.
These templates are also ideal for developing appealing activities in the house or in the class. Easily obtainable and printable, they save time while advertising imagination and productivity. Explore a vast array of designs to meet your special demands today!
Year 5 Spelling Worksheets Printable
Year 5 Spelling Worksheets Printable
These printable worksheets and activities can be used for teaching students the difference between left and right This simple left and right printable worksheet is for preschool/early elementary-age kids. The worksheet also reinforces shape recognition and colors.
Left Right Worksheets All Kids Network

Spelling Worksheets Printable
Year 5 Spelling Worksheets PrintableHelp your child practice recognizing left from right with "Left And Right Shapes" printable worksheet. Your child will color the shape either on the left or on ... Teaching left and right positions using an interactive smart board This was created using Active Inspire Software Very simple look at the pairs of pictures
The game Right or Left for preschool activities can be printed free in A4. Left and right position worksheets are to learn the concepts of «right» and ... 5th Grade Spelling Words Kindergarten Grade Spelling Words
Teaching Left and Right Free Worksheet B Honest Media Pinterest

10 5Th Grade Spelling Worksheets Worksheets Decoomo
Left and right worksheets created just for kindergarteners and first graders Circle cut and glue write left and right and more Printable Spelling Worksheets For 5 Year Olds Thekidsworksheet
Try our left and right worksheets with a variety of visually appealing exercises to identify the position of objects and bolster directional skills Freebie Friday Roll And Spell Printable Spelling Word Practice Printable Spelling Worksheets For Kids Free Worksheets For Kids Free

Spelling Grade 6 Worksheets Pdf

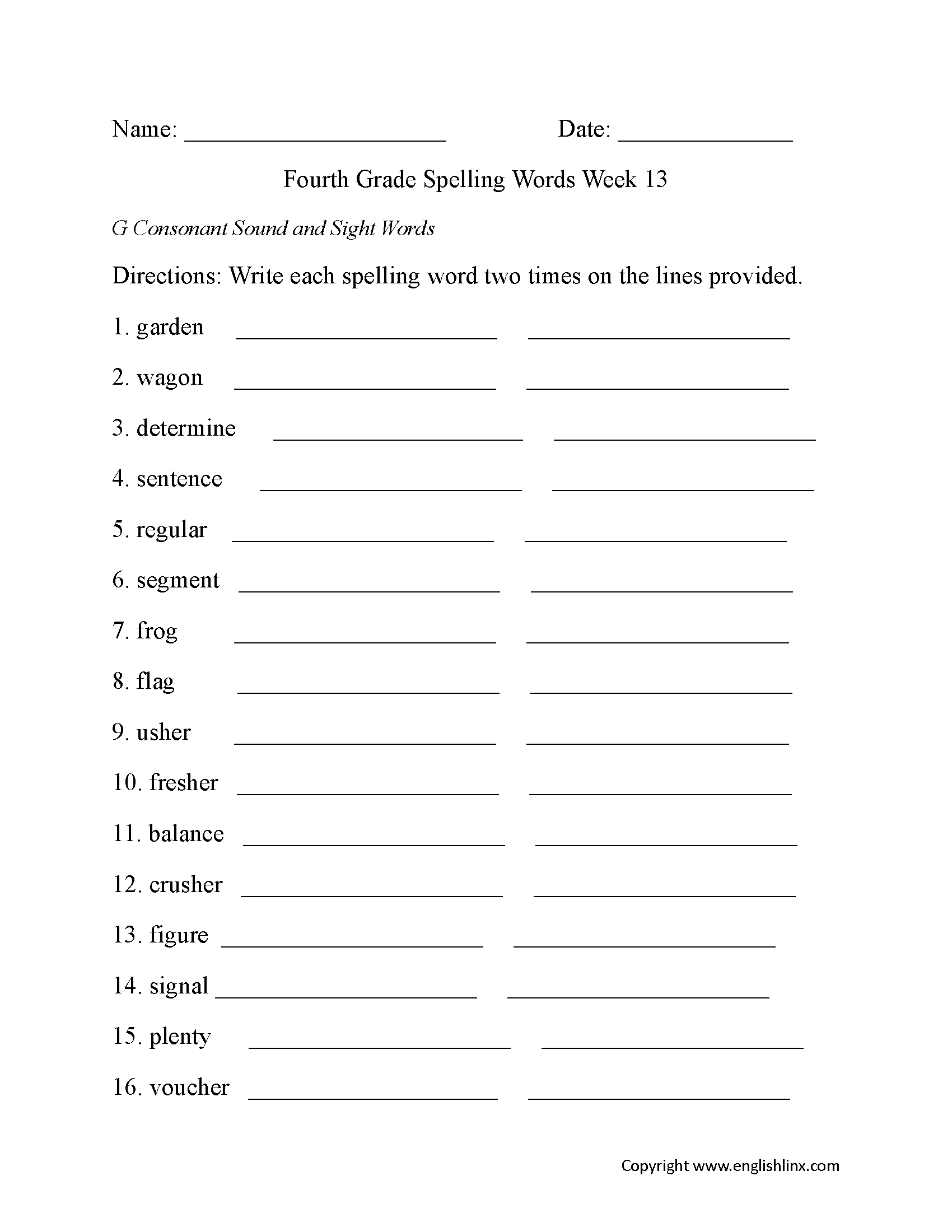
Year 4 Spellings Worksheets Free Download Goodimg co

Number Names Worksheets Pronunciation Worksheets Printable
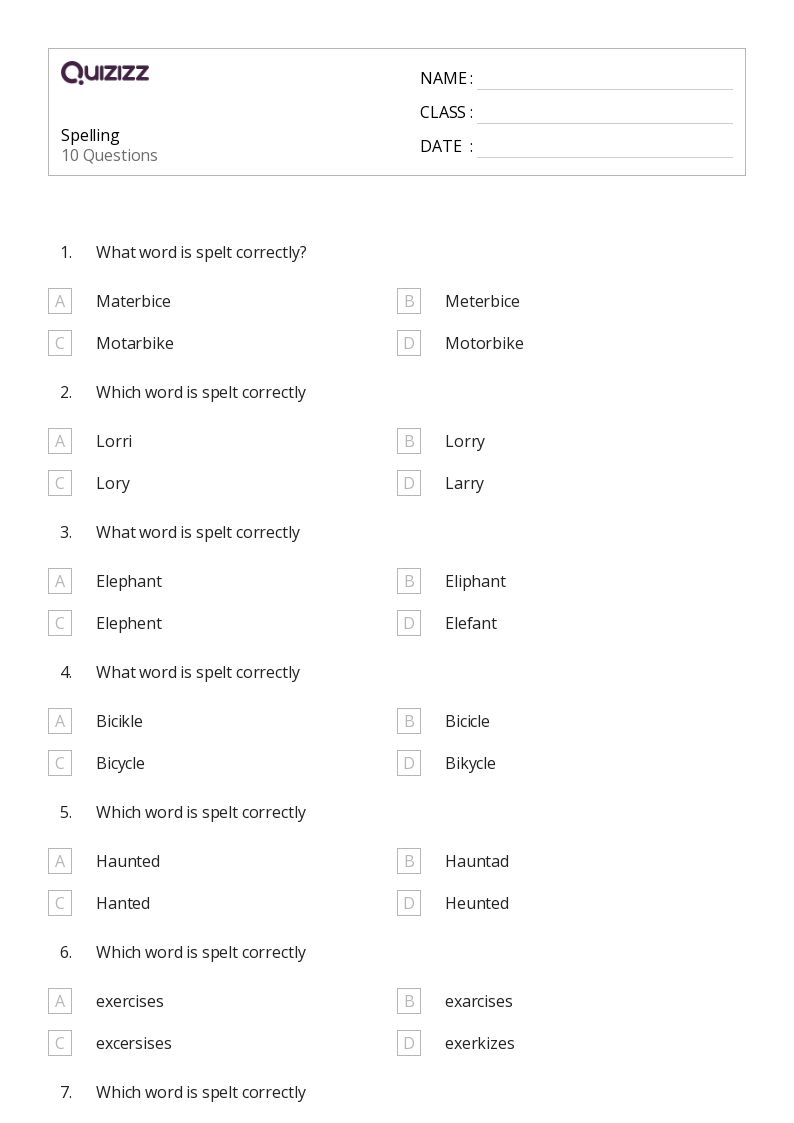
50 Spelling Worksheets For 5th Year On Quizizz Free Printable
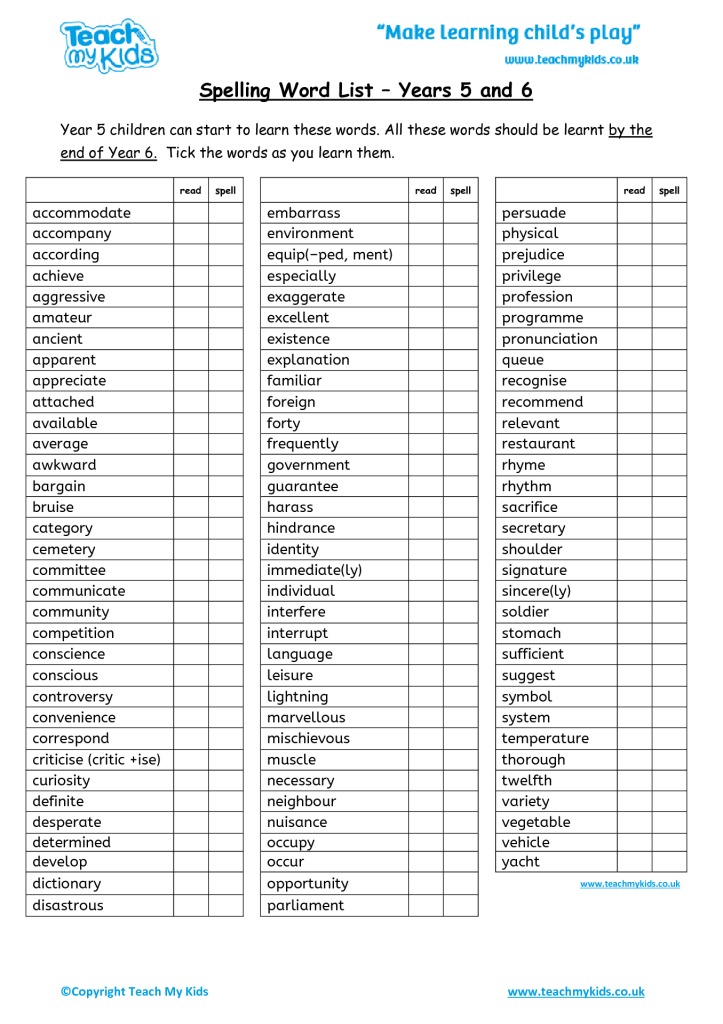
Year 5 Spelling List PDF

List Of Grade 5 Spelling Words GrammarVocab

Printable Spelling Worksheets For 5 Year Olds Thekidsworksheet

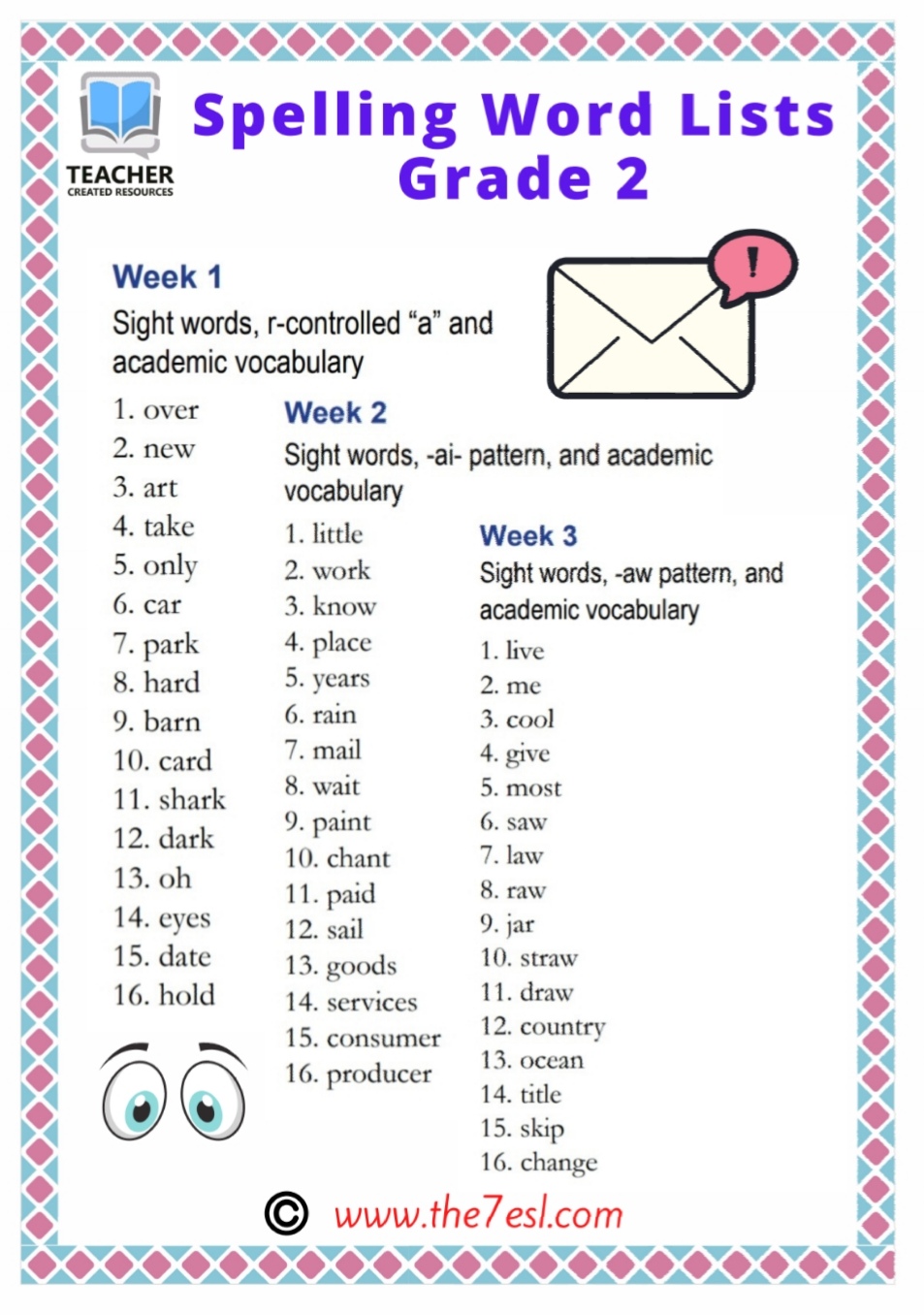
Spelling Word Lists Grade 2