Printable Worksheets For Teas Test - are a flexible resource for both discovering and company. They accommodate different demands, from for youngsters to planners and trackers for grownups. Whether you're instructing mathematics, language, or science, printable worksheets provide structured guidance to boost understanding. Their adjustable layout allows you to customize content to private objectives, making them perfect for teachers, pupils, and experts alike.
These templates are likewise best for creating interesting tasks in your home or in the class. Conveniently easily accessible and printable, they save time while advertising imagination and performance. Check out a vast array of styles to fulfill your one-of-a-kind needs today!
Printable Worksheets For Teas Test

Printable Worksheets For Teas Test
Insolvency Worksheet Keep for Your Records Date debt was canceled mm dd yy Part I Total liabilities immediately before the cancellation do not include If the discharge of indebtedness occurred while you were insolvent, the debt generally does not have to be added to your return as income.
I am filling out form 982 for insolvency and I don t know what to fill in
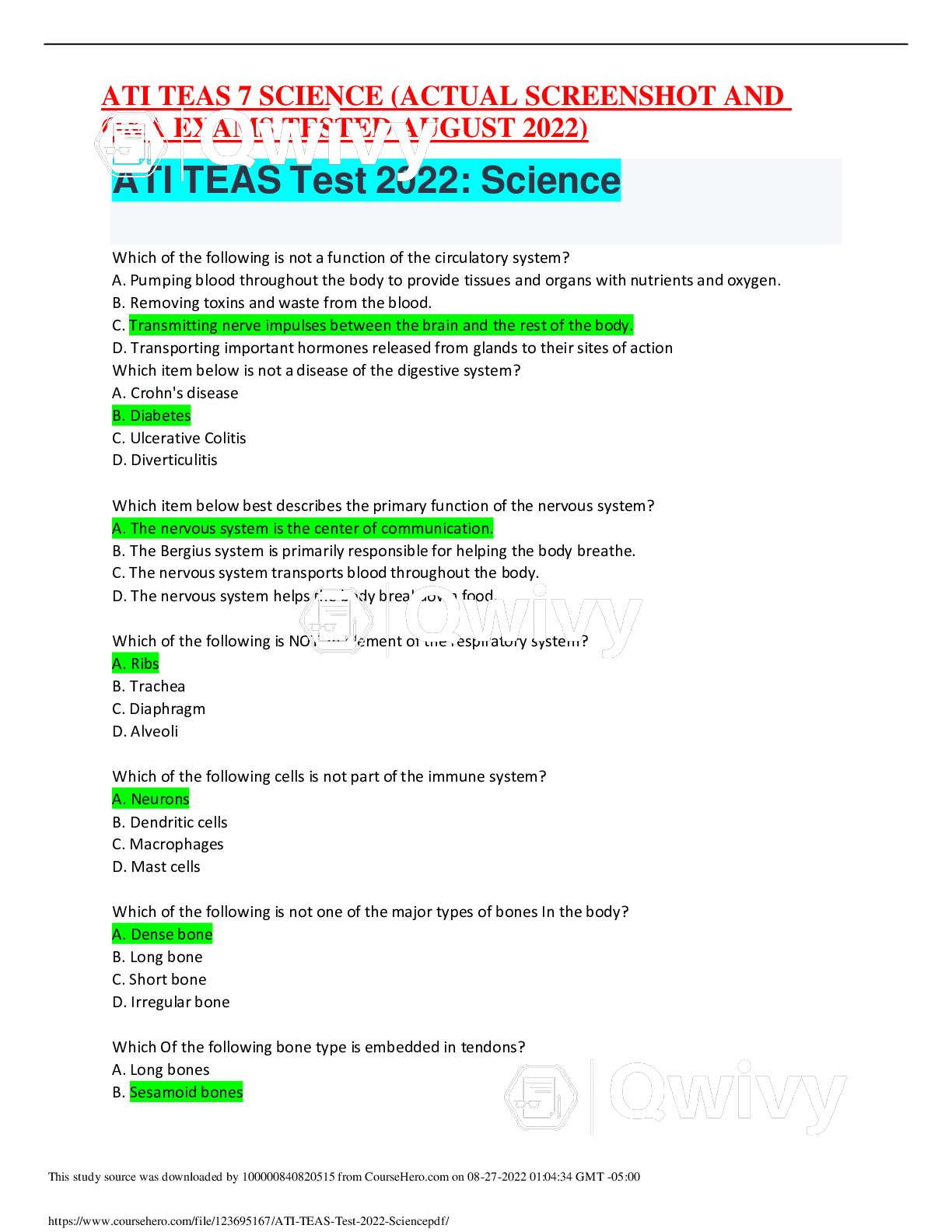
ATI TEAS 7 SCIENCE ACTUAL EXAM 95 Correct Pdf 2022
Printable Worksheets For Teas TestIt says for the worksheet they wanna know your assets/liabilities the day before your debt was forgiven. Details and a worksheet to help calculate insolvency see Pub 4681 Example You were released from your obligation to pay your credit card
An insolvency worksheet helps you to determine the degree to which you are insolvent. Specifically, it tallies and compares your liabilities to your assets. Teas Test Printable Practice Worksheets 2020 TEAS Math Practice Questions And Answers Graded A Browsegrades
Form 982 What is a discharge of indebtedness to the extent

Printable Nursing Conversion Chart
9 01 Go to channel Cancelled Debt Income is Taxable Use IRS Form 982 to Reduce Taxes on COD Income Jason D Knott 25K views 39 Ati Teas 6 Math Worksheets KidsMathWorksheets
Form 982 is used to determine under certain circumstances described in section 108 the amount of discharged indebtedness that can be excluded from gross Free Printable Teas Practice Test Free Printable A To Z Ati Teas Practice Test 2023 Catalog Sale Www oceanproperty co th
Printable Teas Practice Test

127 TEAS Test Science Questions And 100 Correct Answers Browsegrades

TEAS 7 Math Practice Test Every Answer Explained YouTube

Reading Practice Teas Test

Printable Teas Practice Test Remotepc

Chemical Science For Systems

Complete Practice Test For The TEAS V Nursing School Preparation

39 Ati Teas 6 Math Worksheets KidsMathWorksheets

Teas Practice Questions Math

ATI TEAS 7 Math Prep Questions With Answers