Printable Architecture Worksheets For Students - are a functional resource for both discovering and organization. They satisfy various demands, from for children to planners and trackers for grownups. Whether you're educating mathematics, language, or scientific research, printable worksheets offer organized support to improve understanding. Their adjustable format allows you to customize material to specific goals, making them ideal for educators, trainees, and professionals alike.
These templates are also excellent for producing engaging tasks in your home or in the class. Conveniently available and printable, they save time while advertising imagination and productivity. Discover a wide variety of styles to satisfy your special demands today!
Printable Architecture Worksheets For Students

Printable Architecture Worksheets For Students
These free worksheets explore how to determine the meaning of words that have multiple definitions This FREE vocabulary unit provides a variety of ways to work on multiple meaning words or homonyms. It's perfect for upper elementary, middle school, or high ...
Multiple meaning words worksheet hammouti Amazon

Architecture Reading Comprehension Worksheet Games4esl
Printable Architecture Worksheets For StudentsInstantly access Twinkl's printable and digital K-12 teaching resources, including worksheets, eBooks, games, PowerPoints, Google Slides, and more! This no prep worksheet was created to help students practice their multiple meaning goals This product can be printed in color or black
These no prep workbooks will challenge your students with options that include sentence completion, choosing correct usage, and composing original sentences. Gina Wilson All Things Algebra Parallel Lines Online Worksheets Free Printable Boundaries Worksheets For Students Worksheets Library
Free multiple meaning words in sentences TPT
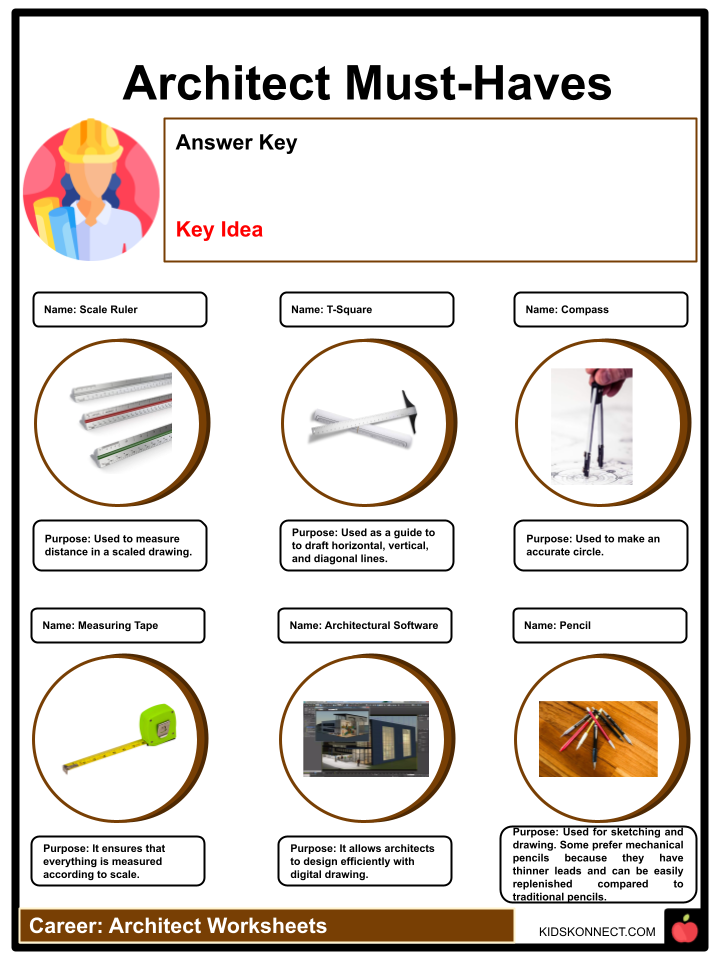
Architect Types Duties Facts Worksheets For Kids
This comprehensive collection of 15 worksheets on multiple word meanings provides students with an enriching and interactive way to explore the complexities of Kids Math Worksheets Free Printable Worksheets Free Printables
In these worksheets students identify two meanings for each homonym These worksheets are available to members only 5 Free Count And Color Kindergarten Worksheets For Students Free Downloadable Activities Let Kids Explore Architecture And Design

Famous Architecture ESL Printable English Vocabulary Worksheets

Architect Types Duties Facts Worksheets For Kids
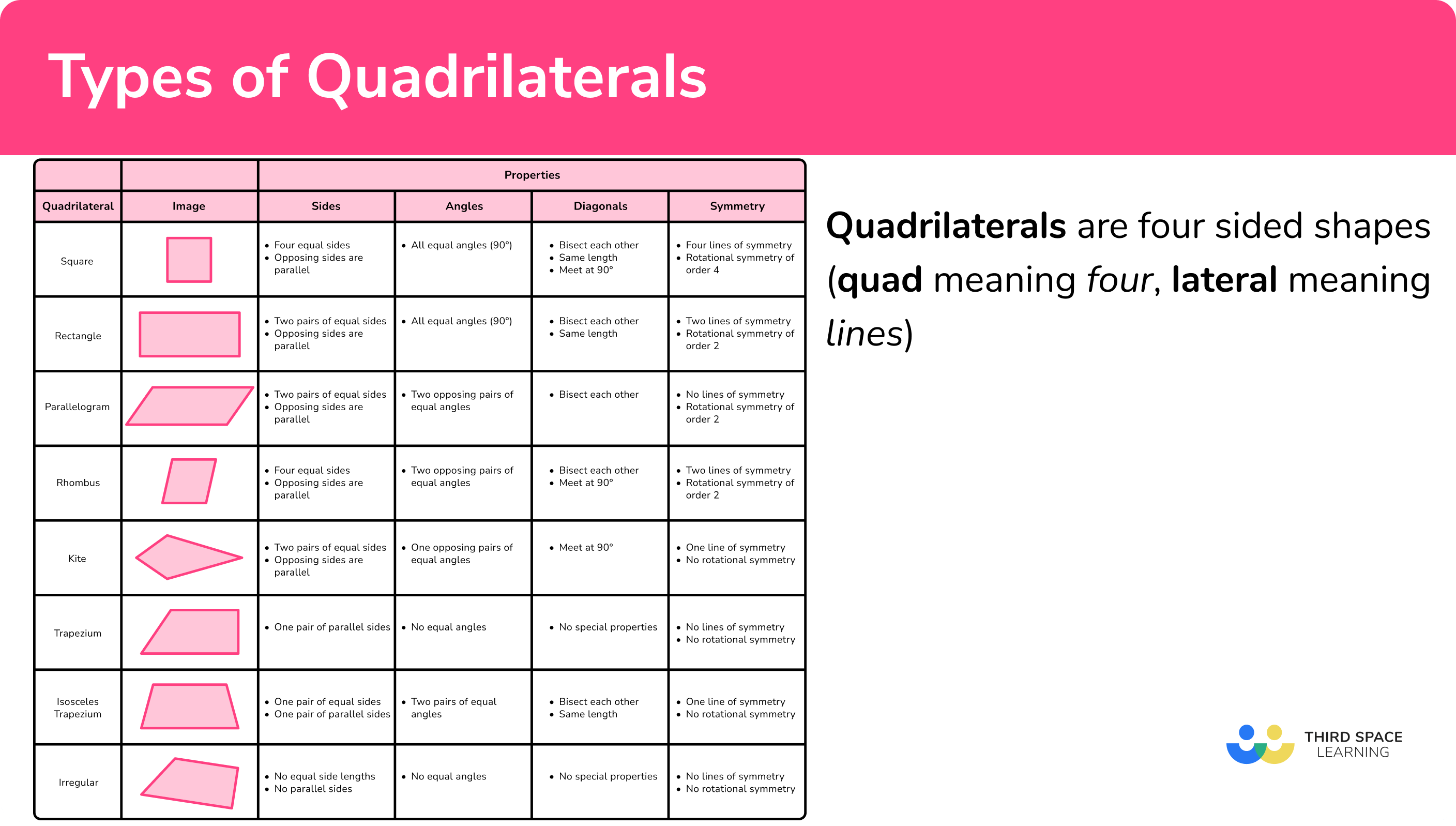
Types Of Quadrilaterals GCSE Maths Steps Examples Worksheets

Architecture Vocabulary Word Search

Ancient Greek Architecture Background Orders Facts Worksheets

Note naming Worksheets Printable FREE Royal Recorders
Worksheet Live Worksheets

Kids Math Worksheets Free Printable Worksheets Free Printables

Architect Types Duties Facts Worksheets For Kids

Famous Architecture ESL Printable English Vocabulary Worksheets