Hands On Equations Printable Worksheets - are a flexible resource for both learning and organization. They cater to various needs, from for children to planners and trackers for grownups. Whether you're teaching math, language, or science, printable worksheets supply structured guidance to enhance understanding. Their personalized layout allows you to customize web content to specific goals, making them excellent for teachers, pupils, and specialists alike.
These templates are additionally ideal for developing appealing activities at home or in the class. Quickly accessible and printable, they save time while promoting creative thinking and productivity. Explore a variety of designs to satisfy your unique requirements today!
Hands On Equations Printable Worksheets
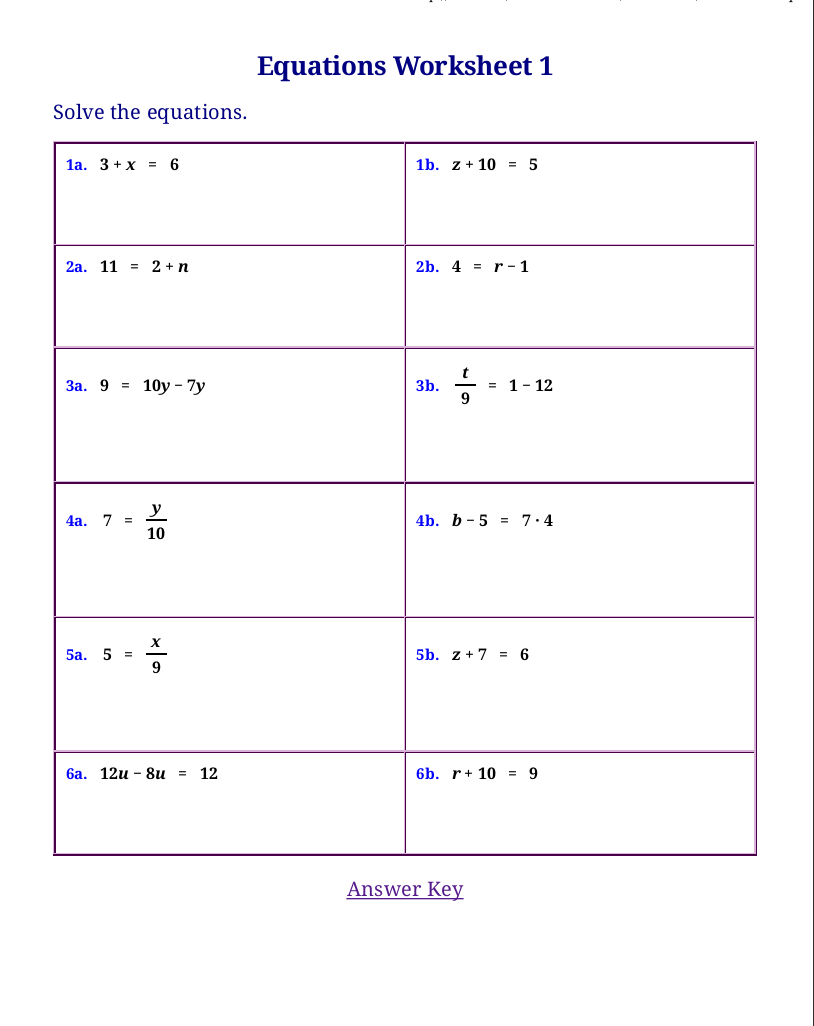
Hands On Equations Printable Worksheets
Printable fall worksheets for kids Check out our collection of printable kids worksheets with a fall theme We have word and picture matching worksheets FREE Fall & Autumn printables and worksheets for toddlers, preschool and kindergarten featuring pumpkins, trees, falling leaves, scarecrows, ...
Fall Printables for Preschoolers Free Printables Packet Pinterest
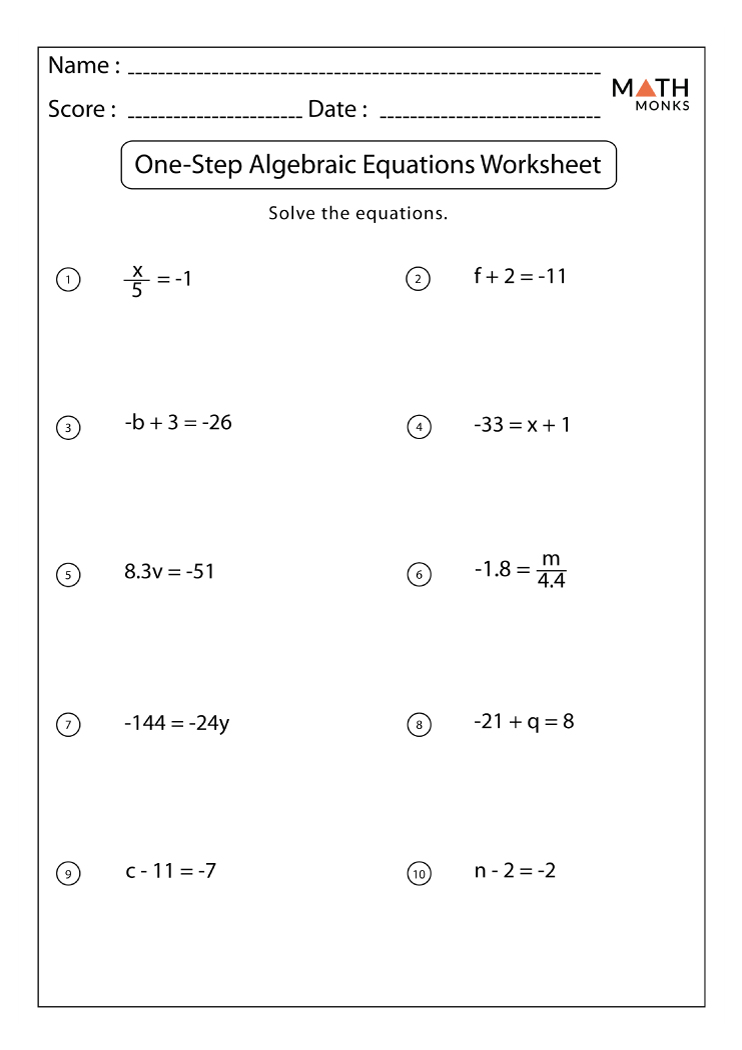
One Step Math Equations
Hands On Equations Printable Worksheets12 free fall preschool worksheets to develop fine motor skills and shape & number recognition. Fall worksheets help your child learn while the leaves are changing Use these fall worksheets to learn about changing seasons fall holidays and more
Autumn alphabeth poster worksheet pre-k printable beginning sound letters busy book fall kids PDF. $3.30. Digital Download. Add to Favorites ... [img_title-17] [img_title-16]
Free Fall Printables for Kids Totschooling Toddler Preschool

Practice Linear Equations Worksheets
Explore a collection of engaging fall themed worksheets for kindergarteners From counting adorable animals amidst autumn scenery to tracing letters [img_title-11]
Fall worksheets help your child learn while the leaves are changing Use these fall worksheets to learn about changing seasons fall holidays and more [img_title-12] [img_title-13]
[img_title-4]
[img_title-5]
[img_title-6]
[img_title-7]
[img_title-8]
[img_title-9]
[img_title-10]
[img_title-11]
[img_title-14]
[img_title-15]