Free Printable Fractions Worksheets No Download - are a versatile source for both learning and organization. They accommodate numerous demands, from for children to planners and trackers for adults. Whether you're showing math, language, or science, printable worksheets give organized guidance to enhance understanding. Their personalized format enables you to customize content to specific goals, making them perfect for educators, students, and specialists alike.
These templates are additionally ideal for creating appealing activities at home or in the class. Conveniently accessible and printable, they save time while advertising creative thinking and productivity. Explore a wide range of styles to satisfy your distinct needs today!
Free Printable Fractions Worksheets No Download

Free Printable Fractions Worksheets No Download
Skeleton Anterior Posterior Cranial Caudal Lateral Left Lateral Right Skull Labeling 1 Select An Answer Students will identify and learn the anatomy of the human skull in anterior, lateral, superior, and inferior views.
Learn skull anatomy with skull bone quizzes and diagrams Kenhub
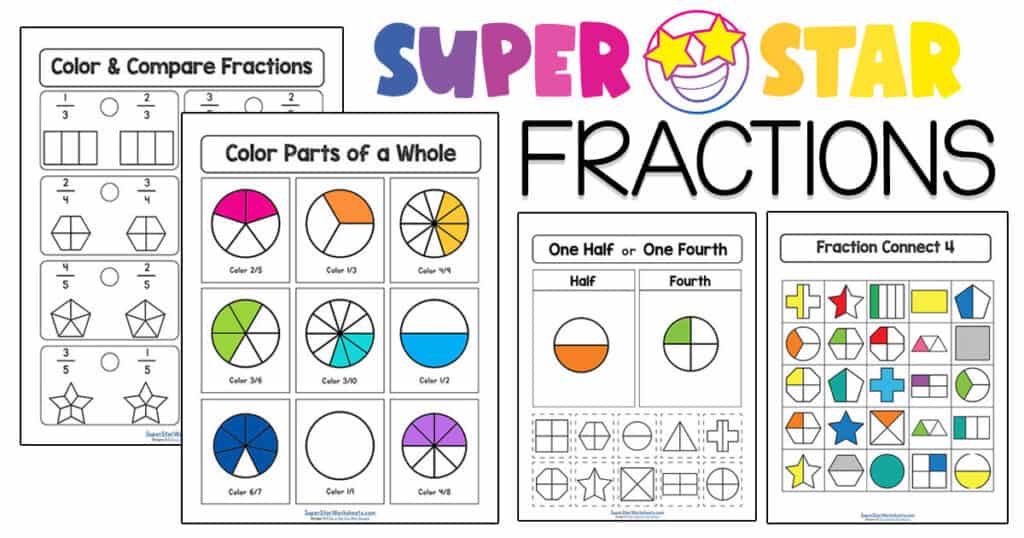
Fractions
Free Printable Fractions Worksheets No DownloadWrite the names of the bones indicated in the drawing below. The dashed lines ( ) indicate 'holes' in the skull, name these structures too. Label the major bones of the skull and color them in As you color in the skull try to use the same color for the same bone on different pages This will help
Are you interested in learning more about human anatomy? This labeling worksheet of the human skull is ideal for both students and aficionados. 50 Class 1 Worksheets On Quizizz Free Printable Time FREE Printable Worksheets Worksheetfun Worksheets Library
Anatomy skulls TPT
FREE Printable Animal Pattern Worksheets For Kindergarten
Skull Anatomy PDF Printable Worksheet Intro to Anatomy Printable PDF Worksheet Skeletal System WorksheetSkull Labeling Activity Skeletal System Biology Maths Fractions Questions
This is a free printable worksheet in PDF format and holds a printable version of the quiz Labeling the Bones of the Skull Fractions Worksheet Grade 5 Math Math Fractions Fractions Worksheets Equivalent Fractions Worksheets 6th Grade

Nutrition Worksheets Free Printable Ideas And Templates Worksheets
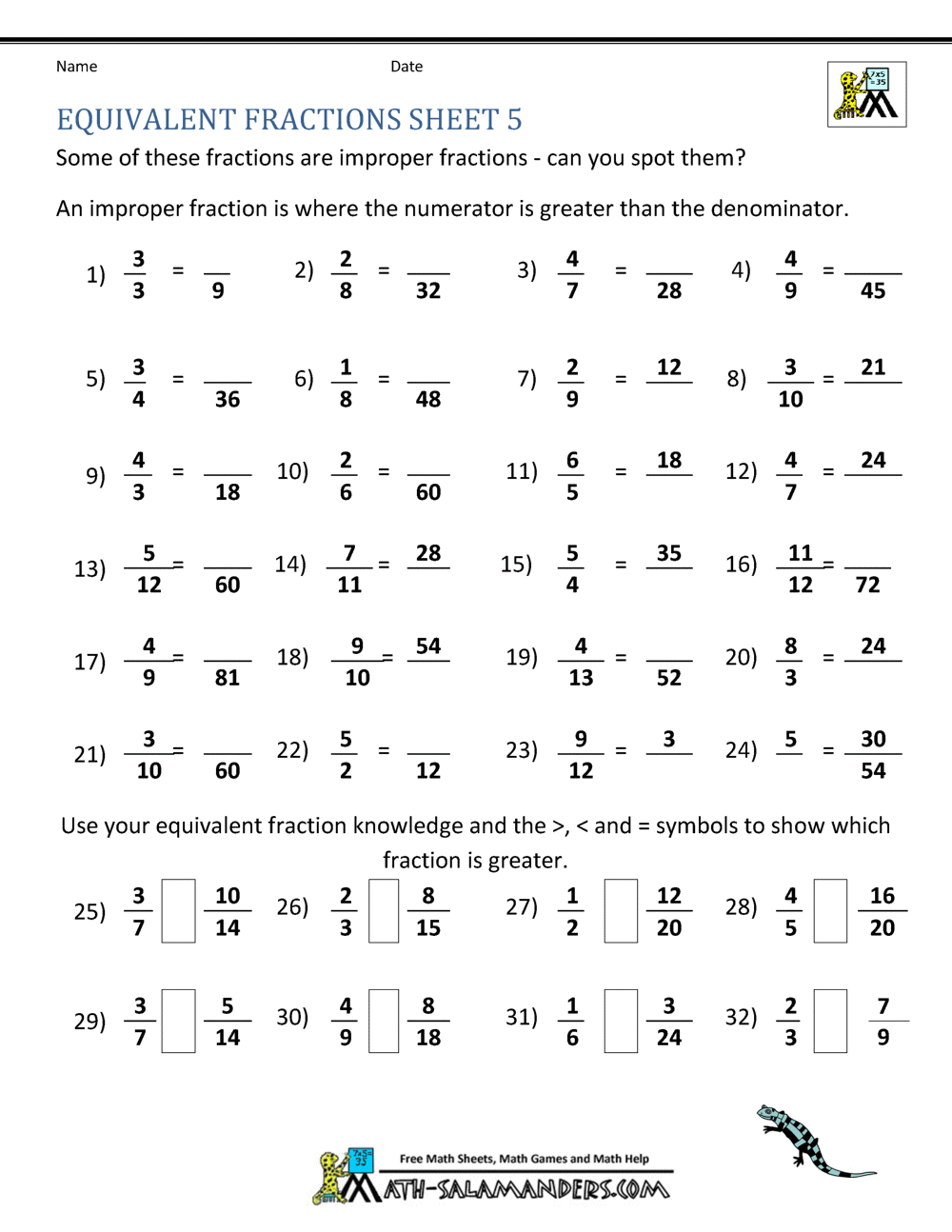
Fractions Worksheets Grade 8 Pdf
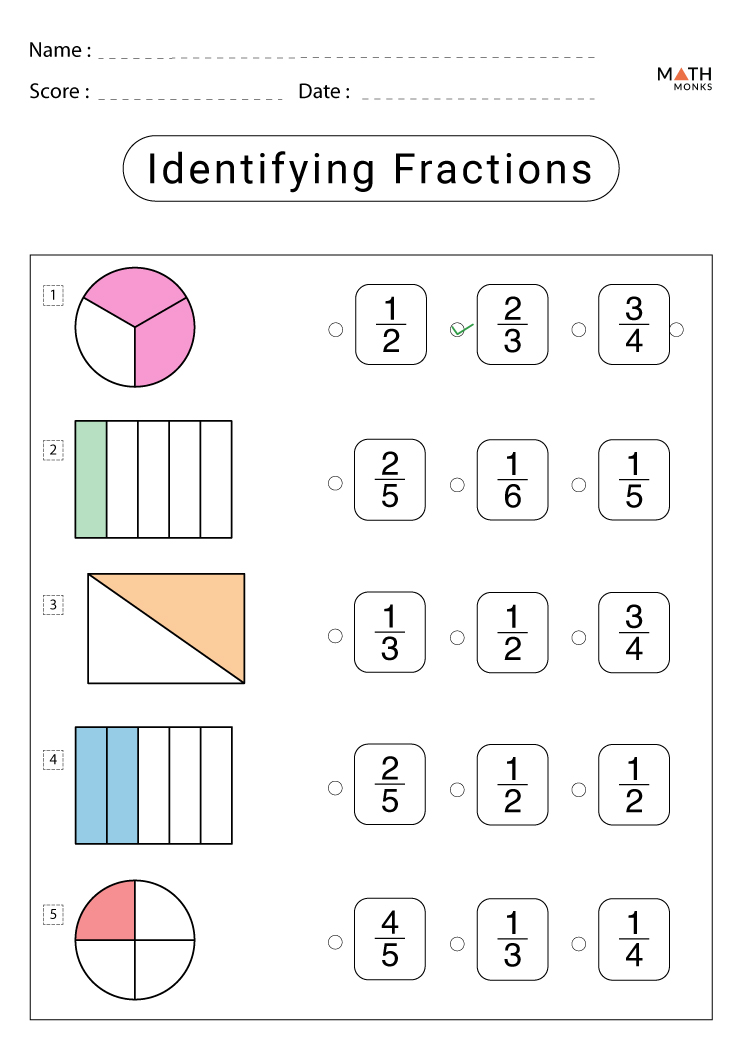
2nd Grade Fraction Worksheets With Answer Key

Fractions Worksheets Grade 6

Addition Worksheets For Fractions

Free Proportions Worksheets Printable Ideas Worksheets Library
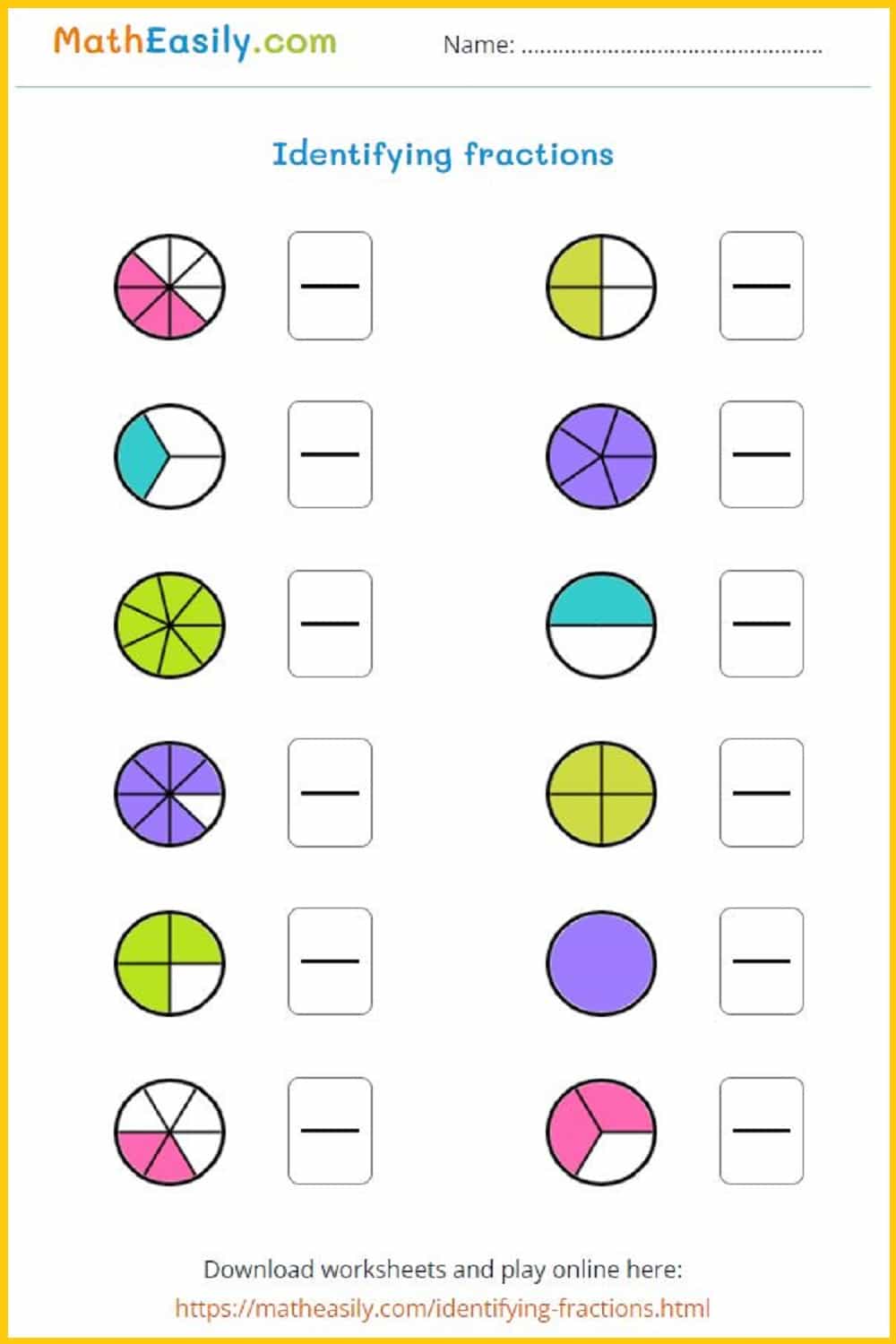
Math Fractions Worksheets PDF Free Download
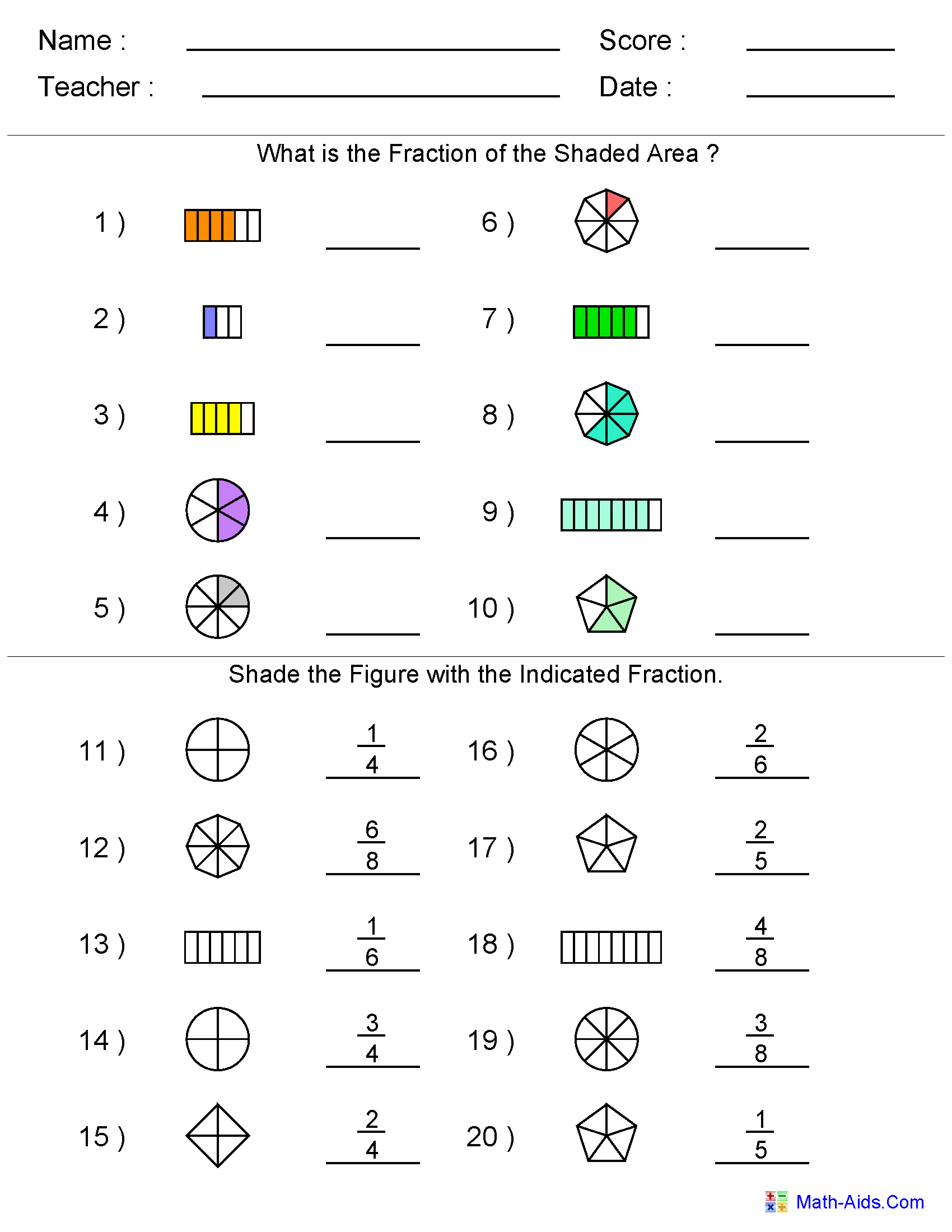
Maths Fractions Questions

34 Pratical Grade Math Notebooks Ideas Math Fractions Worksheets
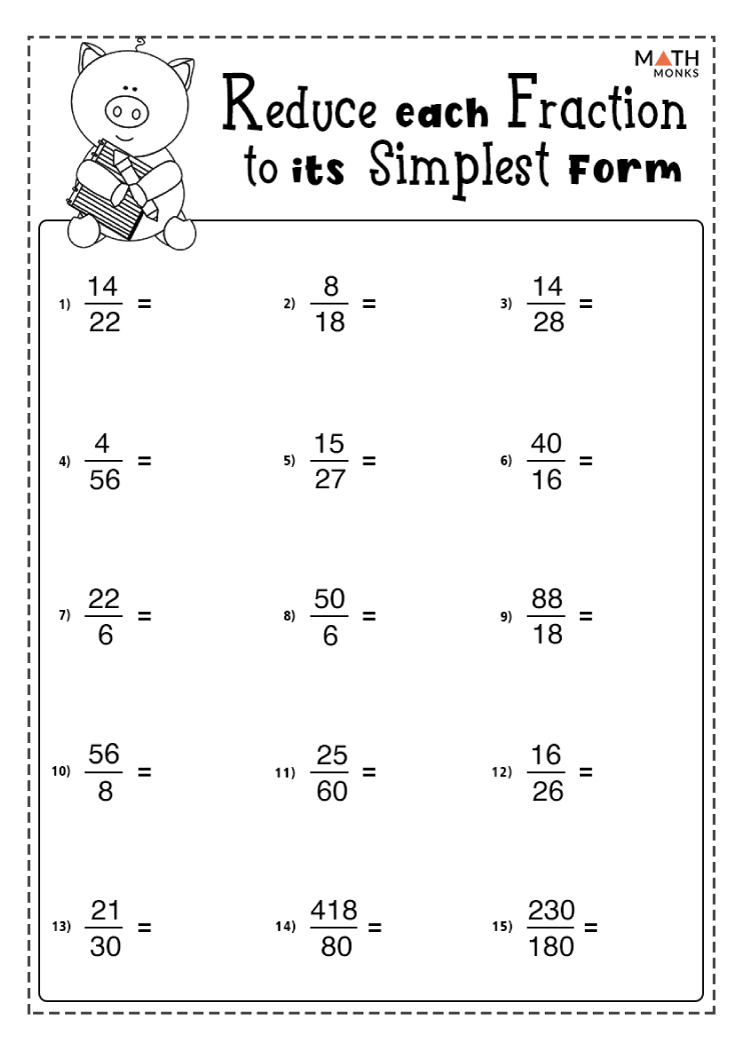
Simplifying Fractions Worksheets Math Monks