Free Printable Book Review Worksheets - are a versatile resource for both learning and company. They cater to different demands, from for children to planners and trackers for adults. Whether you're instructing math, language, or scientific research, printable worksheets provide structured guidance to enhance understanding. Their customizable style allows you to customize material to specific objectives, making them excellent for educators, students, and experts alike.
These templates are additionally ideal for producing engaging activities at home or in the class. Conveniently available and printable, they save time while promoting imagination and productivity. Explore a wide variety of layouts to meet your one-of-a-kind demands today!
Free Printable Book Review Worksheets

Free Printable Book Review Worksheets
This activity teaches students to recognize nonverbal cues and the messages they send Students will consider whether their interpretation of nonverbal Identify a range of non-verbal communication methods. Body language. Worksheet to download, This worksheet introduces students to non-verbal communication.
Free non verbal communication TPT

Book Review Form For Kids The Activity Mom
Free Printable Book Review WorksheetsBrowse our awesome range of teacher-created Nonverbal Communication Worksheets to help your kids communicate their needs and feelings. Find 10 pictures of celebrities expressing distinct emotions on their faces or with their body language Ask the students to number their papers 1 10 Tell
This series of 15 worksheets is specifically designed to help students improve their communication abilities by exploring both verbal and nonverbal aspects of ... Printable Book Review Cards Printable Reading Journal Book Etsy 7 Best Images Of Book Review Printable Template Book Review Template
Non Verbal Communications Resource List fess ie
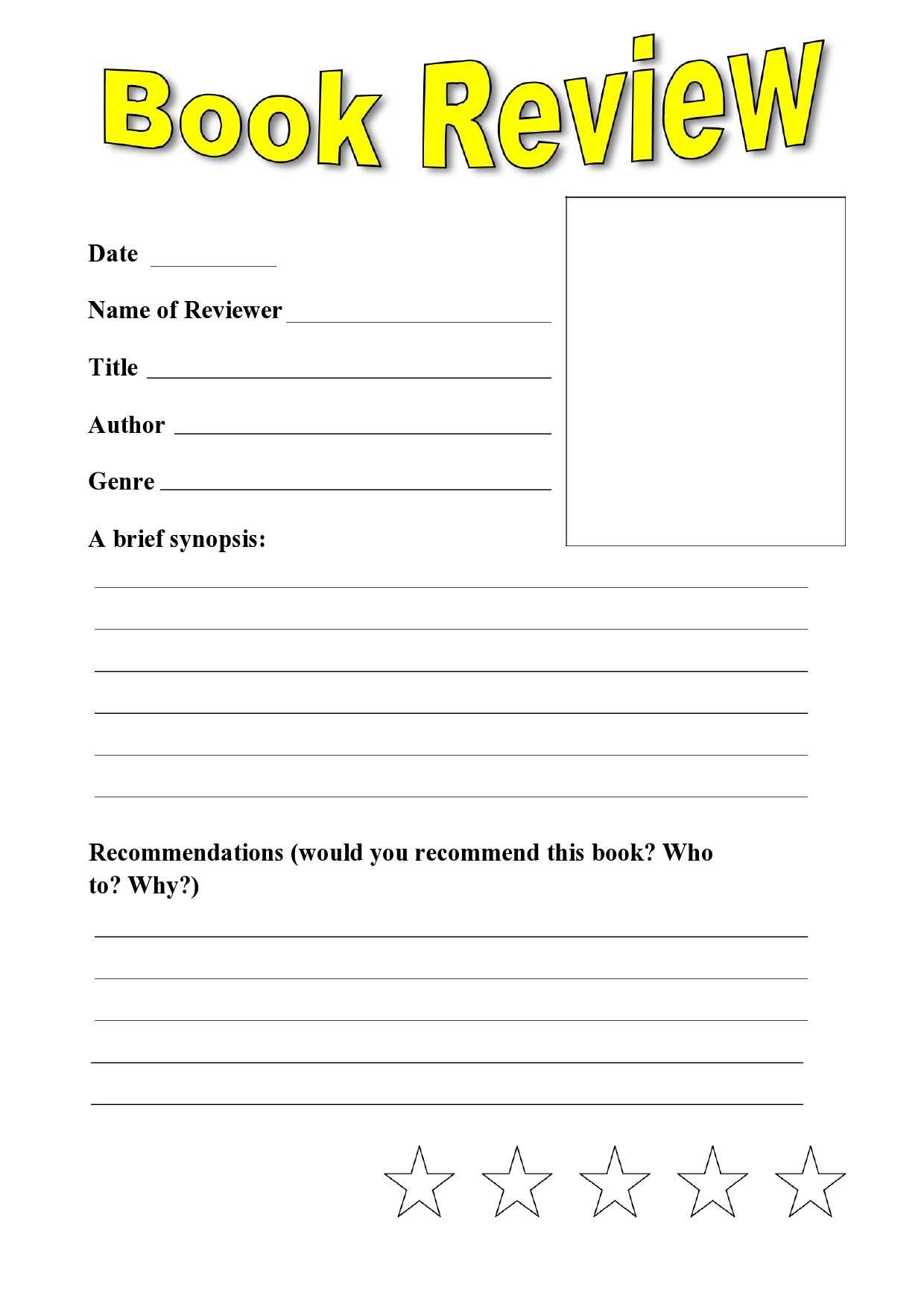
Printable Book Review Template
Identify some of the causes of ineffective communication C Identify forms of nonverbal communication D Describe why communication is important MODULE 21 The Charming 7Th Grade Book Report Outline Education Book Report
Distribute the Non Verbal Communication Worksheet to all students and review the instructions on how to complete it Review the examples provided on the sheet Sandwich Book Report Printable Template Thegreenerleithsocial Printable Book Review Template

Free Printable Children s Book Template Of Free Printable Blank Name

Book Review Template For Kids FREE Shining Brains

The Marvellous Free Printable Book Report Forms Teaching Ideas Book

Pin On Filigrana

Free My Book Review Worksheet BookLife

Book Review English Worksheet WordUnited

My Book Report Printable Worksheet Free Printable Papercraft Templates

The Charming 7Th Grade Book Report Outline Education Book Report

Free Printable Book Review Worksheets Free Templates Printable

Bnute Productions Free Printable Kids Book Report Worksheet