Free Printable 10 Commandments Printable Worksheets - are a versatile source for both knowing and organization. They cater to different requirements, from educational activities for kids to planners and trackers for adults. Whether you're showing math, language, or scientific research, printable worksheets offer structured assistance to enhance understanding. Their adjustable style enables you to customize web content to individual goals, making them perfect for instructors, pupils, and experts alike.
These templates are also excellent for creating engaging tasks in your home or in the class. Easily accessible and printable, they conserve time while advertising creativity and efficiency. Check out a large range of layouts to satisfy your distinct demands today!
Free Printable 10 Commandments Printable Worksheets

Free Printable 10 Commandments Printable Worksheets
What are the 10 Commandments in simple terms 1 Put God first 2 No fake gods 3 Respect God s name 4 Respect God s day of rest 5 Respect · Teach your 5-10 year olds about the Ten Commandments with this free printable Bible lesson pack from Trueway Kids! Includes lesson guides, worksheets, coloring pages, crafts and more to make your lessons engaging and.
Ten Commandment Worksheets Christian Preschool

Ten Commandments Printable Craft Kids Bible Teacher
Free Printable 10 Commandments Printable Worksheets · Help kids have fun learning the Ten Commandments and memorizing their order with some Ten Commandments printable activities. Our Ten Commandments Printable Pack includes copywork, drawing, matching, and. These free printable 10 Commandments worksheets provide a convenient and accessible tool to educate and engage individuals in understanding the principles and guidelines for moral conduct making the learning experience
This is a printable set of ten commandments flashcards for children to use to work on memorizing the ten commandments. Use these as sequencing cards, or pair them with the Ten Commandment Number Cards (below) to make a fun. Printable Catholic 10 Commandments Printable Word Searches Printable 10 Commandments Printable Worksheets
The Ten Commandments 5 10 Year Old Bible Lesson

Free 10 Commandments Printables
Learn the ten commandments for kids by crafting a vibrant and captivating free printable cootie catcher This lively 10 commandments activity effortlessly engages kindergartners grade 1 grade 2 grade 3 grade 4 grade 5 FREE Printable 10 Commandments Colouring Page Colouring Sheets Worksheets Library
The Ten Commandments are written in a simple way easy to understand great for 14 Best Images Of Free Printable 10 Commandments Worksheets Free Printable Ten Commandments Ten Commandment Worksheets Christian Preschool Printables

Ten Commandments Printable Activities

14 Best Images Of Free Printable 10 Commandments Worksheets Free Printable Ten Commandments

Passover Worksheet Ten Commandments Planerium Worksheets Library
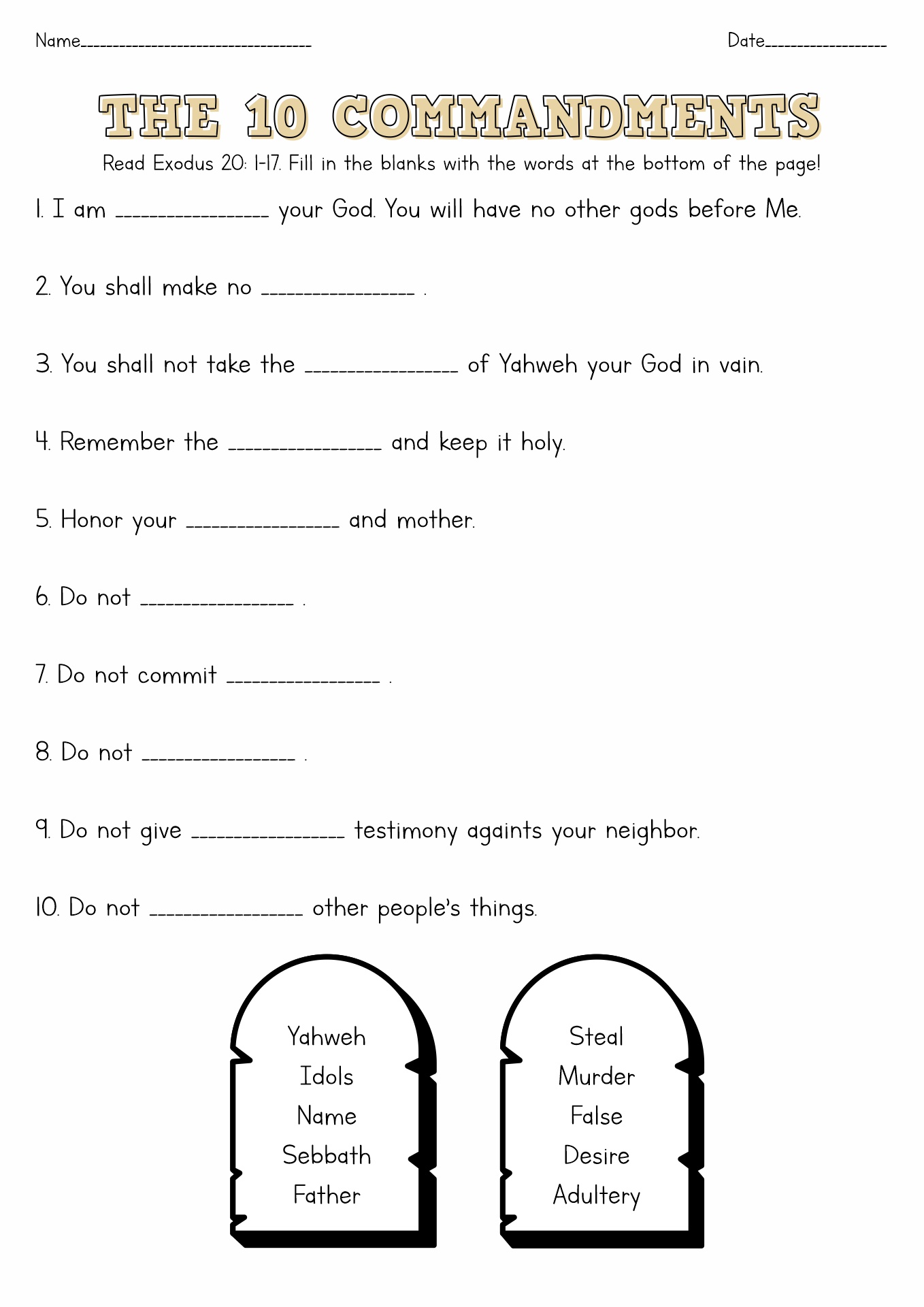
15 Free Printable 10 Commandments Worksheets Free PDF At Worksheeto
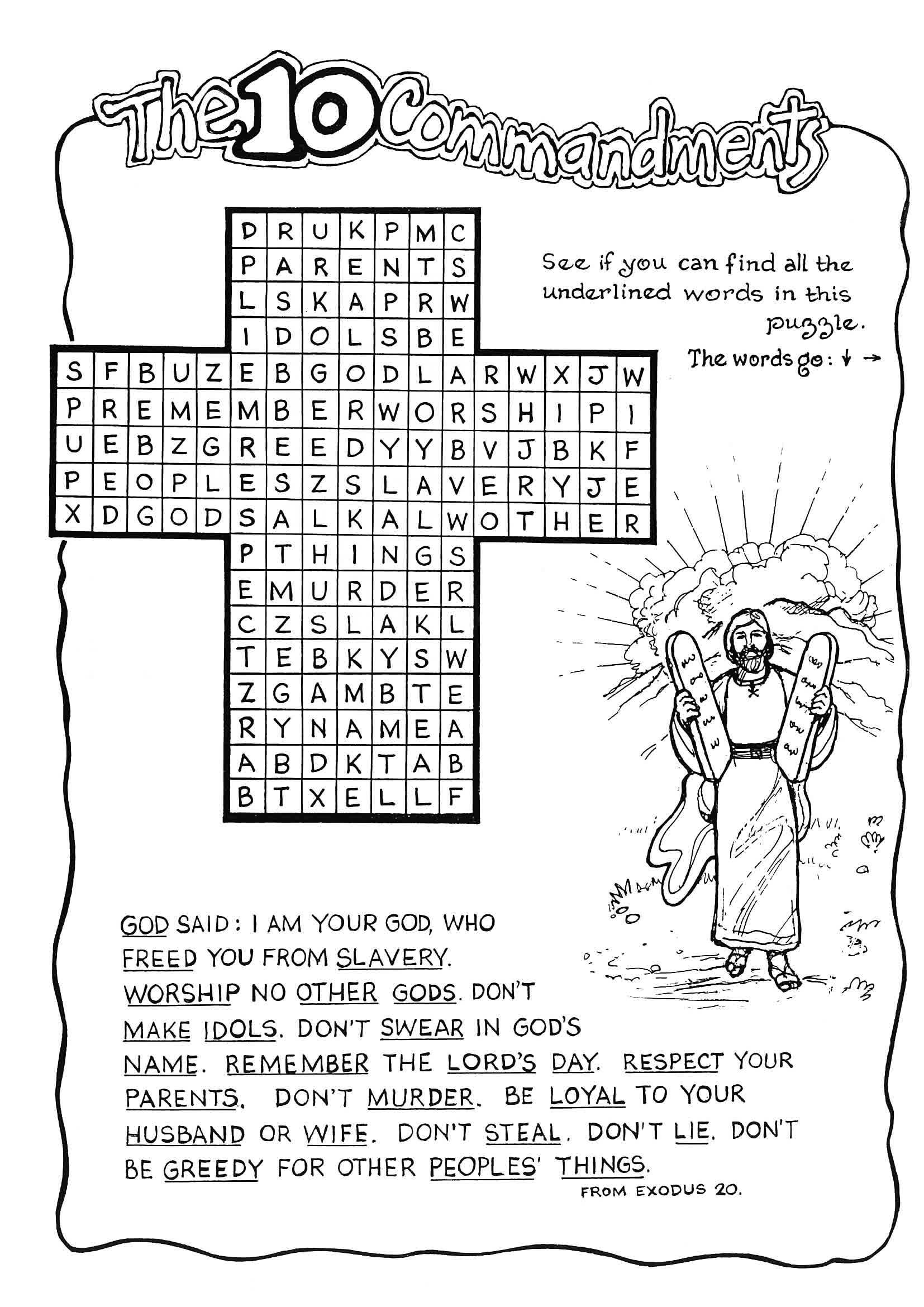
Free Printable Ten Commandments Worksheets Free Printable Templates

15 Free Printable 10 Commandments Worksheets Free PDF At Worksheeto

Ten Commandments Free Printable Worksheets Library

FREE Printable 10 Commandments Colouring Page Colouring Sheets Worksheets Library

15 Free Printable 10 Commandments Worksheets Free PDF At Worksheeto
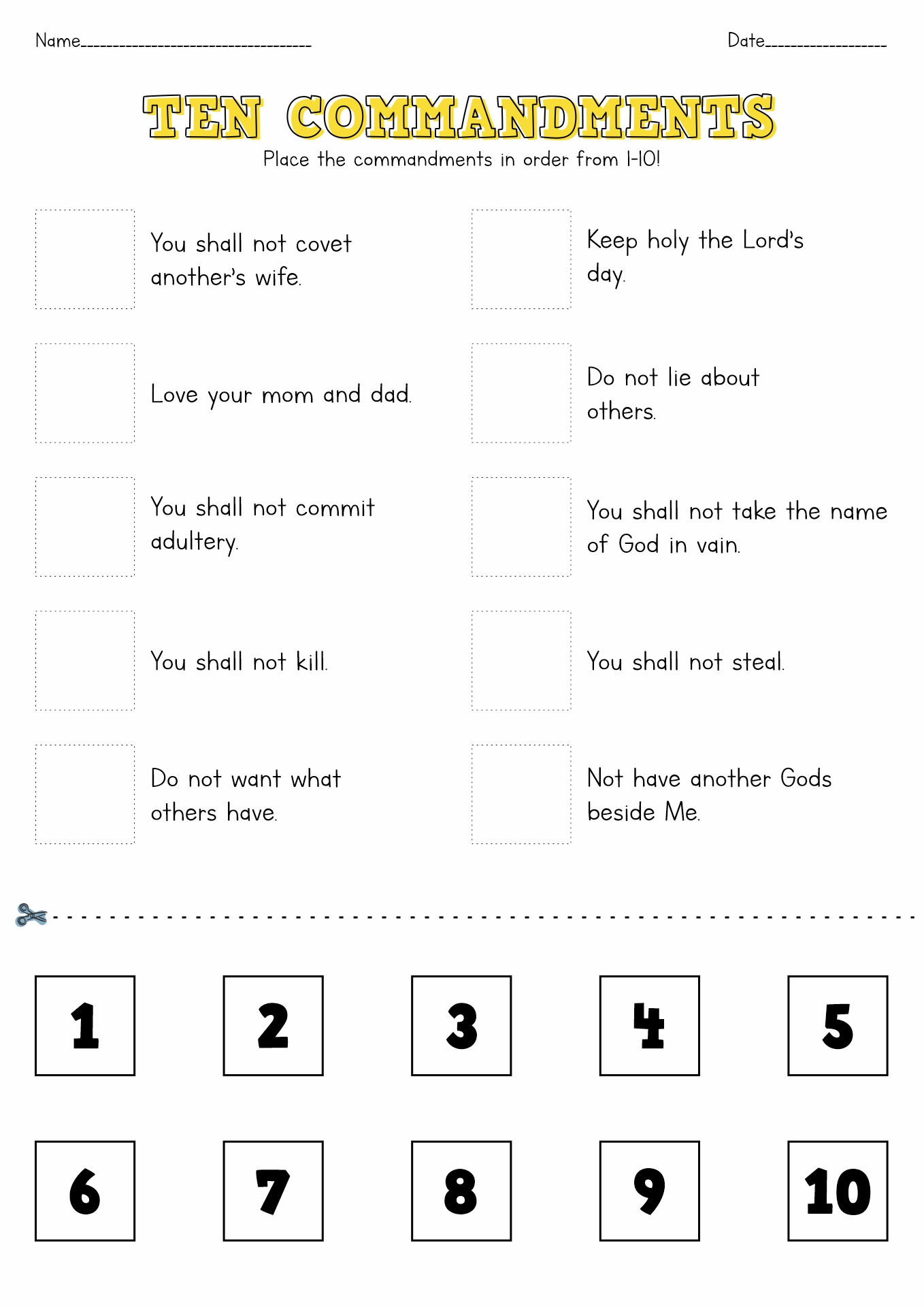
15 Free Printable 10 Commandments Worksheets Free PDF At Worksheeto