English Worksheets For Grade 7 Free Printables - are a versatile source for both discovering and company. They satisfy numerous demands, from for kids to planners and trackers for adults. Whether you're teaching mathematics, language, or science, printable worksheets provide structured assistance to enhance understanding. Their personalized style permits you to customize web content to private objectives, making them perfect for instructors, pupils, and professionals alike.
These templates are additionally excellent for creating interesting activities at home or in the classroom. Easily available and printable, they save time while advertising creativity and productivity. Check out a vast array of layouts to meet your one-of-a-kind demands today!
English Worksheets For Grade 7 Free Printables

English Worksheets For Grade 7 Free Printables
2 digit multiplication Multiplication practice with all factors being under 100 column form Multiply 2 x 2 digits worksheet Open PDF Here you will find a wide range of free multiplication worksheets, which will help your child learn to multiply a range of numbers up to 3 digits by 2 digits.
Multiply 2 digit numbers in columns K5 Learning
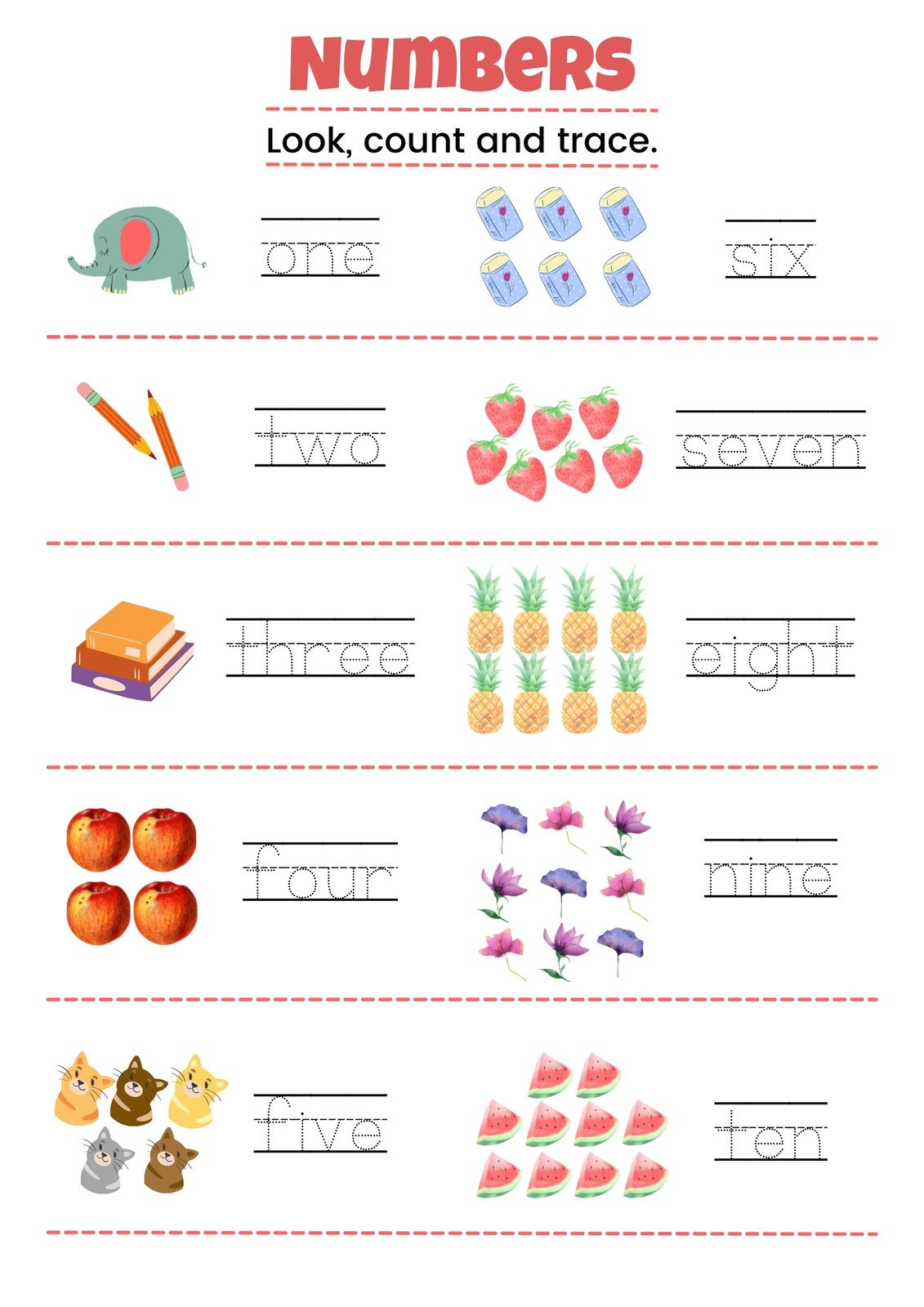
ESL Worksheets English Exercises Worksheets Library
English Worksheets For Grade 7 Free PrintablesPractice double-digit multiplication with various resources including worksheets, games, and digital activities. Multiple brands offer ... We have a hundreds of single and multi digit multiplication printables Includes task cards free worksheets math games word problems and more
Practical Daily Math Practice: Our easy tear-off double-digit math worksheets include 50 pages of exercises; Made for grades 3 to 5; This tool complements 3rd and 4th grade math workbooks, aiding daily multiplication practice for elementary students. Free Printable English Worksheets For Grade 1 Worksheets Library First Grade Worksheets Spelling Worksheets Kindergarten Worksheets
Double Digit Multiplication Worksheets 4th Grade Math Salamanders

Grade 1 English Worksheet Comprehension Smartkids Worksheets Library
This FREE worksheet covers 2 Digit by 1 Digit Multiplication There is room for students to show their work and an answer key is provided for easy grading Grade 5 Printable Worksheets English Worksheet Resume Template
This Multiplication worksheet may be configured for 2 3 or 4 digit multiplicands being multiplied by 1 2 or 3 digit multipliers 20 Grade 6 English Worksheets Worksheets Decoomo Grade 1 Grammar Worksheets Grammar Worksheets Worksheets Free

English Worksheets For Grade 1 Kids Worksheets For All Subjects And Grades

20 Grade 6 English Worksheets Worksheets Decoomo

How To Create English Worksheets For Grade One YouTube

English Worksheets For Kids English Lessons For Kids 1st Grade

Esl Grammar Grammar Online Worksheets For Grade 3 English Worksheets

Printable Fun English Worksheets For Kids To Adults Learning

Pin On English Worksheets

Grade 5 Printable Worksheets English Worksheet Resume Template

Kids English English Lessons Learn English English English Primary
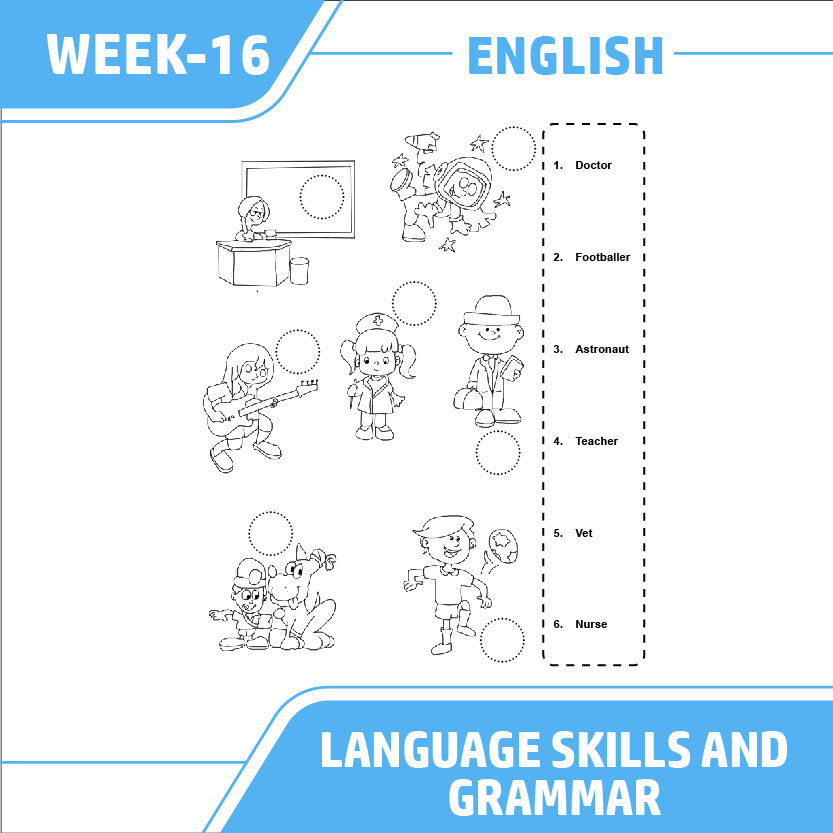
Free Printable English Worksheets Kiddoworksheets Worksheets Library